Prescribing the Future of
Medication Adherence Solutions
AARDEX Group's medication adherence solutions have earned the trust of top pharmaceutical companies worldwide for their accuracy in measuring adherence in clinical trials. With cutting-edge proprietary statistical algorithms at the core of our medication adherence software, MEMS AS®, we provide a powerful combination of analytical capabilities, sleek dashboards, and user-friendly medication adherence packaging and devices, setting the gold standard for adherence monitoring.

ADHERENCE IS POOR IN CLINICAL TRIALS
The Industry's Most Trusted Medication
Adherence Solution
Clinical trials are essential for ascertaining how new treatments will perform in target populations, and reliable data is critical for assessing the effectiveness of new treatments accurately. Unfortunately, non-adherence is a pervasive issue in clinical trials, with up to 50% of participants failing to take their medication as prescribed. Non-adherence not only compromises participants' health outcomes but also undermines the integrity of the trial itself.
As a leading authority on the subject, Pfizer's former Chief Medical Advisor, Dr. Freda C. Lewis-Hall, emphasizes that non-adherence in clinical trials can obscure vital safety signals and significantly complicate the execution of the trial. Therefore, it is imperative to have effective medication adherence solutions in place.
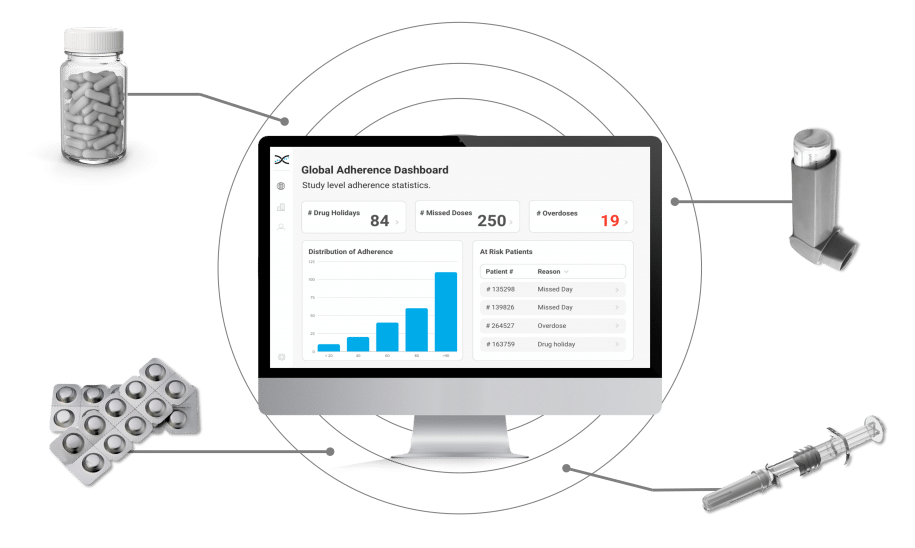
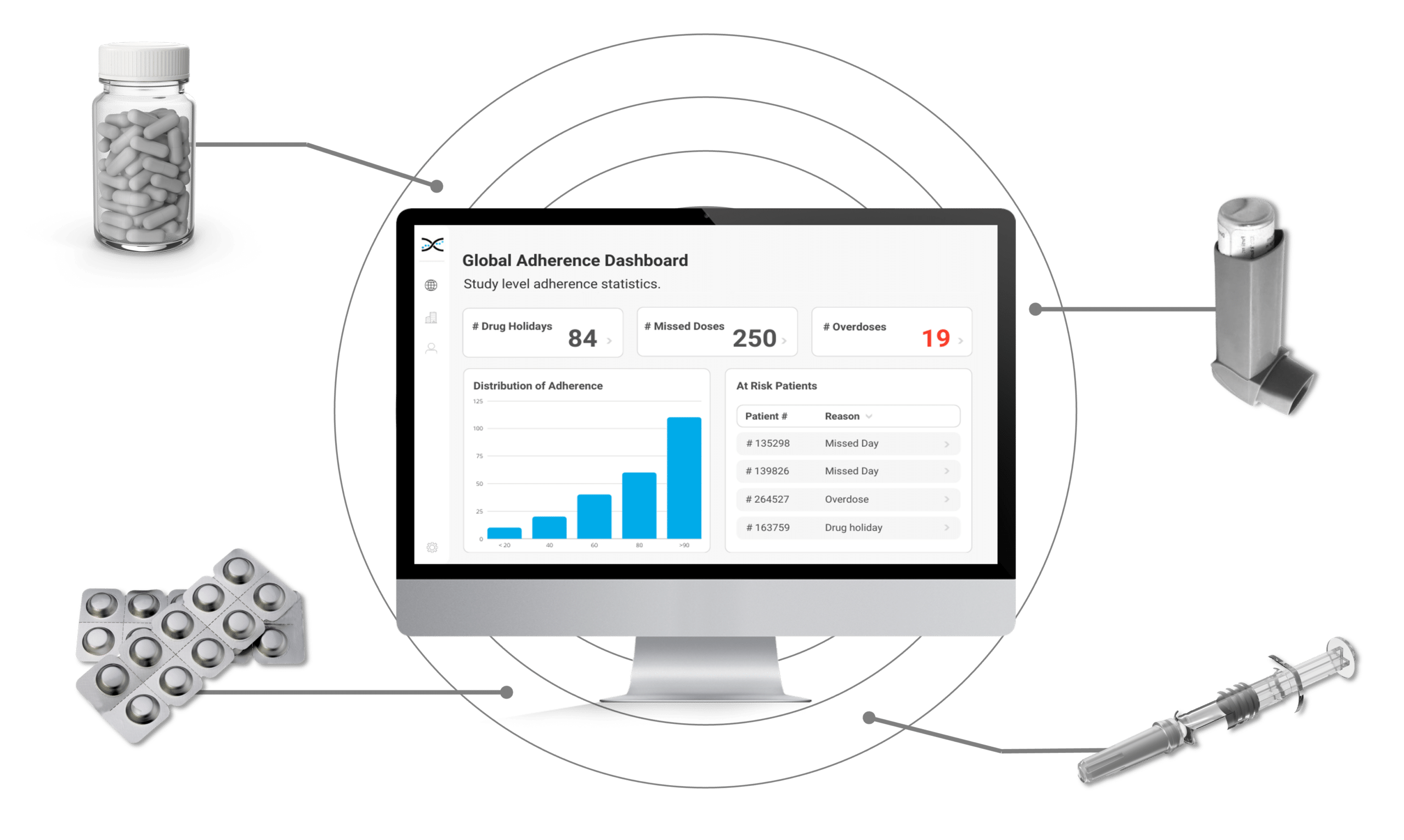
Enter AARDEX Group's Medication Adherence Solution, MEMS AS, a cutting-edge technology powered by advanced algorithms that detect erratic dosing patterns, such as missed doses, drug holidays, and overdoses. MEMS AS alerts investigators when such patterns are detected, prompting focused conversations with participants to maintain adherence. Investigators benefit from valuable insights into medication adherence patterns that can inform future research, ensuring participants take their medication as prescribed.

FEATURES
Real Time Medication Adherence Solution.
Our secure, cloud-based medication adherence solution, MEMS AS, is perfect for researchers that require real-time insights. Driven by over seventy proprietary algorithms, MEMS AS interrogates the medication event data from digitally enabled adherence packaging and devices to provide powerful dashboard visualizations highlighting missed days and drug holidays. With our medication solutions, researchers have accurate insights to identify participants that need additional coaching and support.
- Fully Scalable
- ISO27001 Certified Datacenter
- HIPAA & GDPR Compliant
- IRT, EDC & DCT Integration

FEATURES
Measure Adherence with Precision.
Our medication adherence solution provides unparalleled accuracy and many other features that set it apart from other adherence monitoring methods, such as pill count, self-report, and PK sampling. Research has demonstrated MEMS to be 97% accurate at tracking and collecting participant medication events, while PK sampling is only 70% accurate, pill count at 60%, and self-report at 27%. With MEMS AS in their toolkit, researchers can rest assured that they have access to precise and accurate data to underpin safety and efficacy calculations.
- Mature Solution
- Used in 200+ Drug Trials
- Over 1Mil participants monitored
- Cited in 800+ Papers

FEATURES
Suitable for all Routes of Administration.
Ensuring medication adherence is vital for all medication and delivery methods. To address this issue, we have created an all-encompassing medication adherence solution that utilizes easy-to-use adherence packaging and a diverse network of partners to provide solutions for all routes of administration, including oral dose drugs, injectables, inhalables, and beyond. Our impressive roster of partners includes some of the industry's most innovative and highly regarded companies, enabling us to offer a comprehensive medication adherence solution.
- Participant Acceptability High
- User-Friendly Solutions

FEATURES
Improve Medication Adherence.
Removing barriers to adherence in trials is critical to achieving the best possible participant outcomes and preserving research integrity. Our user-friendly adherence packaging and devices place no burden on participants, only asking that they take their medications. Moreover, participants benefit from access to MEMS® Mobile, where they can schedule medication reminders, helping them stay on track.
- User-Friendly App
- IoS & Android Compatible
- Available in 25 Languages
- 20K+ Users in 30 Countries

FEATURES
Approach Backed by the FDA.
The guidance of the FDA's "Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products" is a clear call to action for the research industry to prioritize medication adherence. The FDA is advocating for the use of medication adherence packaging to encourage participants to take their medications as prescribed.
- ICH GCP Compliant
- FDA 21 CFR part 11 Compliant

OUR CLIENTS
The Go-To Solution for Pharma Companies
Some of the world's leading pharmaceutical companies have embraced our medication adherence solutions. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Medication Adherence Packaging →
Discover our range of medication adherence packaging.

Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Frequently Asked Questions
Medication adherence is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure medication adherence is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Why is adherence to medication important in trials?
Adherence to medication is crucial in clinical trials because it directly affects the integrity and validity of the study results. Clinical trials are designed to evaluate the safety and efficacy of new drugs, treatments, or interventions in a controlled and systematic manner. Therefore, study participants must take their medication as prescribed to ensure the trial results are accurate and reliable.
Poor medication adherence can lead to several issues in clinical trials. For example, it may result in incomplete or inconsistent data, which can compromise the study’s statistical power and reduce the ability to detect significant differences between the treatment groups. Additionally, non-adherence can lead to biased results, as patients who do not take their medication as prescribed may have different outcomes than those who do, even if they are in the same treatment group.
To minimize the impact of poor adherence, clinical trial protocols typically include measures to promote and monitor medication adherence, such as providing education and counseling. However, while education and counseling can certainly promote better adherence, in clinical trials with many participants, getting to the participants that require additional support can be challenging. That’s where AARDEX Group’s solutions come into play. Our medication adherence solution continuously monitors participant medication events to guide researchers to the participants that need support. Inherent stratification lists provide a ranked view of participants prioritized by the participants with problematic behaviors (missed days, drug holidays, overdoses, etc.)
By ensuring high levels of medication adherence, clinical trials can produce robust and accurate results, which are essential for making informed decisions about the safety and effectiveness of new treatments.
What factors affect adherence?
Adherence in clinical trials can be influenced by a variety of factors, including:
Patient-related factors: Patients’ health beliefs, attitudes, motivation, and perceived benefits and risks of the medication can affect their willingness to adhere to the medication regimen.
Patients’ understanding of the trial protocol, the medication’s purpose, and their social and cultural background can also play a role.
Treatment-related factors: The medication’s dosing frequency, route of administration, side effects, and regimen complexity can affect medication adherence. If patients experience side effects or find the medication regimen difficult to follow, they may be less likely to adhere to the prescribed regimen.
Study-related factors: The trial length, the study design, the blinding status, and the study setting can affect medication adherence. Patients enrolled in longer trials or trials with more frequent visits may be more likely to experience adherence issues due to the inconvenience and time commitment.
To address these factors, clinical trials may employ various strategies to promote medication adherence, such as patient education and counseling, simplified dosing regimens, side effect management, reminder systems, and supportive care. By identifying and addressing the factors that affect medication adherence in clinical trials, researchers can optimize the study design and improve the validity and reliability of the trial results.
What factors improve adherence?
Improving medication adherence in clinical trials can be challenging, but several strategies effectively promote adherence. Some factors that can improve medication adherence in clinical trials include:
Patient education and counseling: Providing patients with clear and concise information about the medication’s purpose, dosing instructions, and potential side effects can help increase their understanding and motivation to adhere to the regimen.
Simplified dosing regimens: Simplifying the medication regimen by reducing the dosing frequency, minimizing the number of pills or injections, and using combination products can help make the regimen easier to follow and reduce the likelihood of missed doses.
Reminder systems: Using reminder systems such as phone calls, text messages, or electronic reminders can help patients remember to take their medication and improve adherence.
Supportive care: Providing patients access to healthcare providers, social workers, or support groups can help them cope with the challenges of adhering to the medication regimen and provide additional resources and support.
Adequate monitoring and feedback: Regular monitoring of medication adherence and providing patients with feedback on their adherence can help improve their adherence to the medication regimen.
Patient-centered care: Considering patients’ preferences, cultural beliefs, and social circumstances can help tailor the medication regimen to their individual needs and improve adherence.
By employing these strategies, clinical trial investigators can improve medication adherence and increase the accuracy and reliability of the study results.
What are the methods to measure adherence?
Several methods are used to measure medication adherence in clinical trials, each with strengths and weaknesses. Here are some common methods, sorted by accuracy.
Electronic monitoring: This involves using either medication adherence packaging or devices that include hidden sensors or microprocessors that automatically capture and store the date and time the medication is removed/administered. The value of this type of approach is that it is non-invasive and does not add a burden for participants. Capturing this type of data is critical to understand how participants are taking their meds. And that is easy to understand because by using an electronic monitoring system combined with sophisticated remote monitoring software, like AARDEX Group’s adherence software, MEMS AS, sponsors do not need to interrogate medication event data – that’s done by over seventy algorithms that are used to detect problematic behaviors. (97% accuracy rating)
Biological markers: This involves measuring drug levels in a patient’s blood or urine samples. This method provides objective and accurate information on medication adherence. However, it is expensive and may be affected by factors such as drug metabolism and individual variability. Moreover, due to the sampling frequency, the results are sparse and only represent the participant’s adherence in the days leading up to the visit. (70% accuracy rating)
Pill counts involve counting the number of pills or doses patients return at each study visit. This method is relatively easy to administer and requires no special equipment. However, it relies on patients’ honesty in returning all the medication and may not detect missed doses. (60% accuracy rating)
Self-report: This involves patients reporting their medication adherence through questionnaires or interviews. This method is easy to administer and inexpensive. However, it is subjective and may be influenced by patients’ social desirability bias. Moreover, it adds a significant burden for participants. (27% accuracy rating)
Each of these methods has its strengths and limitations, and the choice of the method should depend on the specific requirements of the trial and the resources available. Combining multiple methods can provide a more comprehensive assessment of medication adherence.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.


Collaborating for Safer, More Efficient Trials.
By combining technology and partnerships, we are revolutionizing how medication adherence is monitored in clinical trials. Our unique adherence ecosystem brings together leading medication adherence packaging and devices and DCT, IRT, and EDC vendors, CROs, and CMOs to drive innovation.
