Injay Pre-Filled Syringe Add-On
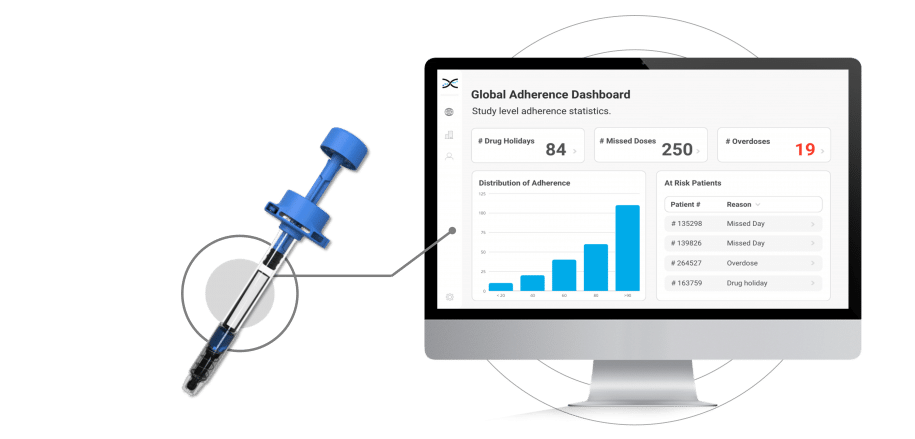
The Injay, developed by Biocorp, is a pre-filled syringe add-on designed to identify non-adherence to injectable drugs. Equipped with NFC tag technology, the Injay captures the date and time of drug administration. Drug administration information is then synchronized with AARDEX Group's medication adherence software, MEMS AS®, providing researchers real-time visualizations on medication adherence. With the Injay and MEMS AS, sponsors can identify non-adherence issues early and provide coaching to trial participants to ensure they stay on track with their medication regimen.

INJAY
Don't Let Non-Adherence Compromise Drug
Efficacy Calculations
Non-adherence to prescribed medication is a well-established problem with significant clinical and commercial consequences for all healthcare stakeholders. This problem is not restricted to commercialized drugs and real-world scenarios; it also significantly impacts clinical trials and can compromise the evaluation of drug efficacy.
When it comes to injectable drugs, patients face unique challenges, including difficulties handling the drug delivery device and needle phobia, as well as complex treatment plans that involve multiple injections spaced over time, which can contribute to non-adherence. The challenges are particularly relevant for medication delivered via pre-filled syringes, which are increasingly used in clinical trials, particularly those involving biologics. Although autoinjectors have been developed to address many of the usability issues related to PFS in routine care, they have not been widely implemented during clinical development.
Injay pre-filled syringe for measuring and managing non-adherence
In response to this challenge, Biocorp and AARDEX Group have collaborated to leverage their respective strengths, establishing connectivity between Biocorp's Injay pre-filled syringe and AARDEX Group's medication adherence software. This partnership provides a comprehensive solution for measuring and managing non-adherence to injectable drugs in clinical trials, ensuring that researchers can access vital information to help inform decision-making. By improving medication adherence, Biocorp and AARDEX Group help sponsors improve patient outcomes, reduce research costs, and support the development of effective therapies for patients in need.
Features of the Injay Pre-Filled Syringe

FEATURES
Identify and Manage Non-Adherence.
Coaching is a proven strategy for addressing non-adherence, but without proper context, it can be challenging for researchers to identify participants who need support in a timely manner. Fortunately, the integration of Injay with MEMS AS removes the guesswork from understanding whether participants are adhering to their injection regimen as prescribed. The powerful dashboards provided by MEMS AS display visualizations that show the state of adherence across participants, studies, and sites. This provides researchers with insights into typical non-adherence patterns, such as missed days, drug holidays, and overdoses, allowing them to quickly identify participants who require assistance. By leveraging MEMS AS, researchers can get to the participants who need help faster, keeping them on track with their medication regimen and promoting better health outcomes.
- Fully Scalable
- ISO27001 Certified Datacenter
- HIPAA & GDPR Compliant
- IRT, EDC & DCT Integration
FEATURES
Ensures the Right Dose at the Right Time.
The Injay pre-filled syringe is a smart solution for managing injectable medication. Featuring an activator located in the syringe finger flange that detects a complete injection when the piston rod is pushed down to the stopping point, the Injay enures the correct dose of medication is delivered, reducing the risk of dosing errors. After injection, participants simply scan the NFC tag on the syringe with their cell phone to register the complete injection with a specific time stamp. This information is then linked to the treatment plan and synchronized with MEMS AS, allowing researchers to conveniently monitor adherence to medication.
- Compatible with Standard PFSs
- User-Friendly
- Compatibility with Syringe Safety Systems

FEATURES
Boost Participant Engagement.
MEMS® Mobile is a mobile app designed to help participants stay on top of their dosing regimen. The app is a powerful tool that helps participants develop strong adherence habits by keeping them on time with their medications. Participants can set reminders for specific times of the day when they need to take their medication, ensuring that they do not miss a dose. The reminders can be customized to fit the participant's schedule, making it easier for them to stay on track with their medication regimen. Additionally, the app provides important information about the prescribed dosing regimen. This information helps participants understand the importance of taking their medication as directed.
- User-Friendly App
- IoS & Android Compatible
- Available in 25 Languages
- 20K+ Users in 30 Countries

OUR CLIENTS
The Go-To Solution for Pharma Companies
Some of the world's leading pharmaceutical companies have embraced our medication adherence solutions. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Medication Adherence Packaging →
Discover our range of medication adherence packaging.

Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Frequently Asked Questions
Medication adherence is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure medication adherence is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Pre-filled syringes and autoinjectors are both types of drug delivery devices that are designed to make it easier for patients to self-administer their medication. However, there are some key differences between the two.
Pre-filled syringes are essentially ready-to-use syringes that come pre-filled with the medication. They are typically made of glass or plastic and are sterilized to ensure the safety and efficacy of the medication. The syringe is typically fitted with a needle that can be attached to the syringe before use. Pre-filled syringes are usually designed for single use, and they can be either manually or automatically injected.
Autoinjectors, on the other hand, are self-contained devices that are designed to inject the medication automatically. They typically include a spring-loaded mechanism that injects the medication once the device is triggered. Autoinjectors often come with a pre-attached needle, and they are typically designed for single use.
When it comes to clinical trials, pre-filled syringes are often preferred over autoinjectors for a few reasons. Firstly, pre-filled syringes are simpler to use and require less training than autoinjectors. This makes them more suitable for use in large-scale clinical trials where participants may have varying levels of experience with self-injecting medication.
Secondly, pre-filled syringes are often more cost-effective than autoinjectors, particularly for drugs that are being developed for rare or orphan diseases. Pre-filled syringes can be produced in large quantities and can be easily transported and stored, making them a practical choice for clinical trials.
Lastly, pre-filled syringes offer more flexibility in terms of dosing than autoinjectors. With a pre-filled syringe, the dose can be adjusted easily by changing the volume of medication in the syringe. With an autoinjector, the dose is typically fixed and cannot be adjusted.
Overall, while autoinjectors have their benefits, pre-filled syringes are the more commonly used drug delivery device in clinical trials due to their simplicity, cost-effectiveness, and flexibility.
Critical product information (product reference, concentration, batch number, expiry date, syringe unique ID) are stored on an NFC tag located on the syringe piston rod. This information can be read before injection using a standard reader or a smartphone equipped with NFC-reading capabilities to check the characteristics of the product.
After injection, users scan the data with the NFC reader to register the complete injection with a specific time stamp and link the information with the treatment plan.
In the standard Injay configuration, the NFC tag is located on the syringe piston rod and the activator on the finger flange, which makes it by design compatible with all standard PFSs, regardless of their materials (plastic, glass), needle formats (staked needles, luer lock, luer cone) or size (0.5 mL, 1 mL long and short, 2.25 mL or other specific sizes). But other configurations can be explored to meet specific requirements, such as compatibility with certain safety systems.
From the regulatory standpoint, both EU and US authorities have implemented specific requirements around needlestick protection, EU Directive 2010/32/EU – “Prevention from Sharp Injuries in the Hospital and Healthcare Sector” and the US Needlestick Safety and Prevention Act (2000). From a user standpoint, this issue has become critical, not only in hospital settings, where the frequency and repetition of injections performed by doctors and nurses increase the risks of needlestick injuries, but also for patients delivering their treatment at home, for whom safety has become a primary concern.
Some examples of non-adherence in clinical trials include:
Missing doses: Patients may forget to take their medication as prescribed, resulting in missed doses.
Taking incorrect doses: Patients may take the wrong dose of medication or take the medication at the wrong time.
Discontinuing treatment: Patients may stop taking the medication before the end of the prescribed treatment period.
Altering the treatment regimen: Patients may change the dose or frequency of medication without consulting their healthcare provider or the clinical trial staff.
Failing to follow instructions: Patients may not follow instructions related to the medication or treatment, such as dietary restrictions or lifestyle changes.
Using additional medications: Patients may use additional medications or supplements without consulting their healthcare provider or the clinical trial staff, potentially affecting the study results.
In clinical trials, adherence refers to a patient’s ability to follow the prescribed treatment regimen as instructed by the clinical trial protocol. Adherence means that the patient is following the treatment regimen carefully and as prescribed, including taking the medication as directed, following any dietary or lifestyle changes as required, and attending all necessary appointments or assessments.
On the other hand, non-adherence, or non-compliance, refers to a patient’s failure to follow the prescribed treatment regimen as instructed by the clinical trial staff. This can include missing doses, taking incorrect doses, discontinuing treatment, altering the treatment regimen without consulting healthcare providers, or failing to follow instructions related to the medication or treatment.
There are several factors that can contribute to non-adherence in clinical trials. Some of the most common factors include:
Treatment-related factors: The treatment being tested in a clinical trial may have side effects or require a complicated dosing schedule, making it difficult for patients to adhere to the prescribed regimen.
Patient-related factors: Patients may have personal factors that affect their ability to adhere to the treatment regimen, such as forgetfulness, lack of motivation, or difficulty following complex instructions.
Logistical challenges: Patients may face logistical challenges that make it difficult for them to adhere to the prescribed regimen, such as transportation issues, financial constraints, or time constraints.
Misunderstandings or lack of information: Patients may misunderstand the treatment regimen or lack information about the study requirements, making it difficult for them to adhere to the prescribed regimen.
Fear or mistrust: Some patients may be fearful or mistrustful of the treatment being tested, the healthcare providers or researchers involved, or the clinical trial process in general, leading them to be non-adherent.
Depression or other mental health issues: Patients may experience depression or other mental health issues that make it difficult for them to adhere to the prescribed regimen.
It is important for researchers to understand the factors that contribute to non-adherence in clinical trials and work with patients to address these factors. This may involve providing additional education and support, simplifying the treatment regimen, or addressing logistical challenges.
Non-adherence, or non-compliance, is a common issue in clinical trials. The prevalence of non-adherence can vary depending on several factors, including the type of treatment being tested, the complexity of the treatment regimen, and the patient population being studied.
Studies have shown that non-adherence rates in clinical trials can range from 10% to 80%, depending on the specific trial and patient population.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.


Collaborating for Safer, More Efficient Trials.
By combining technology and partnerships, we are revolutionizing how medication adherence is monitored in clinical trials. Our unique adherence ecosystem brings together leading medication adherence packaging and devices and DCT, IRT, and EDC vendors, CROs, and CMOs to drive innovation.
