In 1920, The Lancet published a paper authored by T.C. Graves entitled ‘Commentary on a case of hystero-epilepsy with delayed puberty: Treated with testicular extract’.
While the article was not the first to mention the placebo effect, it is recognised as the first to define and articulate the phenomenon, with Graves referencing cases where it appeared to have produced a “real psychotherapeutic effect”.1 In the century that has followed its publication, despite medical scientists having continued to investigate this complex concept, it remains shrouded in a degree of mystery.2
One particular aspect of the placebo effect where understanding remains limited is its relationship with adherence. This area was first discovered more than four decades ago by the Coronary Drug Project Research Group (CDPRG). The group’s findings were presented at the 20th Annual Conference on Cardiovascular Disease Epidemiology in San Diego in March 1980 before being published in the New England Journal of Medicine later that year.3
The CDPRG analysis of a randomised controlled trial (RCT) found that among patients taking clobifrate over a five-year period, good adherers – those with an adherence level of at least 80% to the prescribed protocol – had a substantially lower mortality compared with poor adherers. This underlined the association between good adherence to a drug and the likelihood of improved health outcomes.
More surprisingly, the study also uncovered similar findings among the placebo group. Here, those with good adherence to the placebo ‘drug’ were also associated with improved mortality rates in comparison with poor adherers. This finding led the authors to highlight the difficulty in evaluating the efficacy of the treatment in question among patients grouped according to their exposure to the treatment specified in the research protocol.
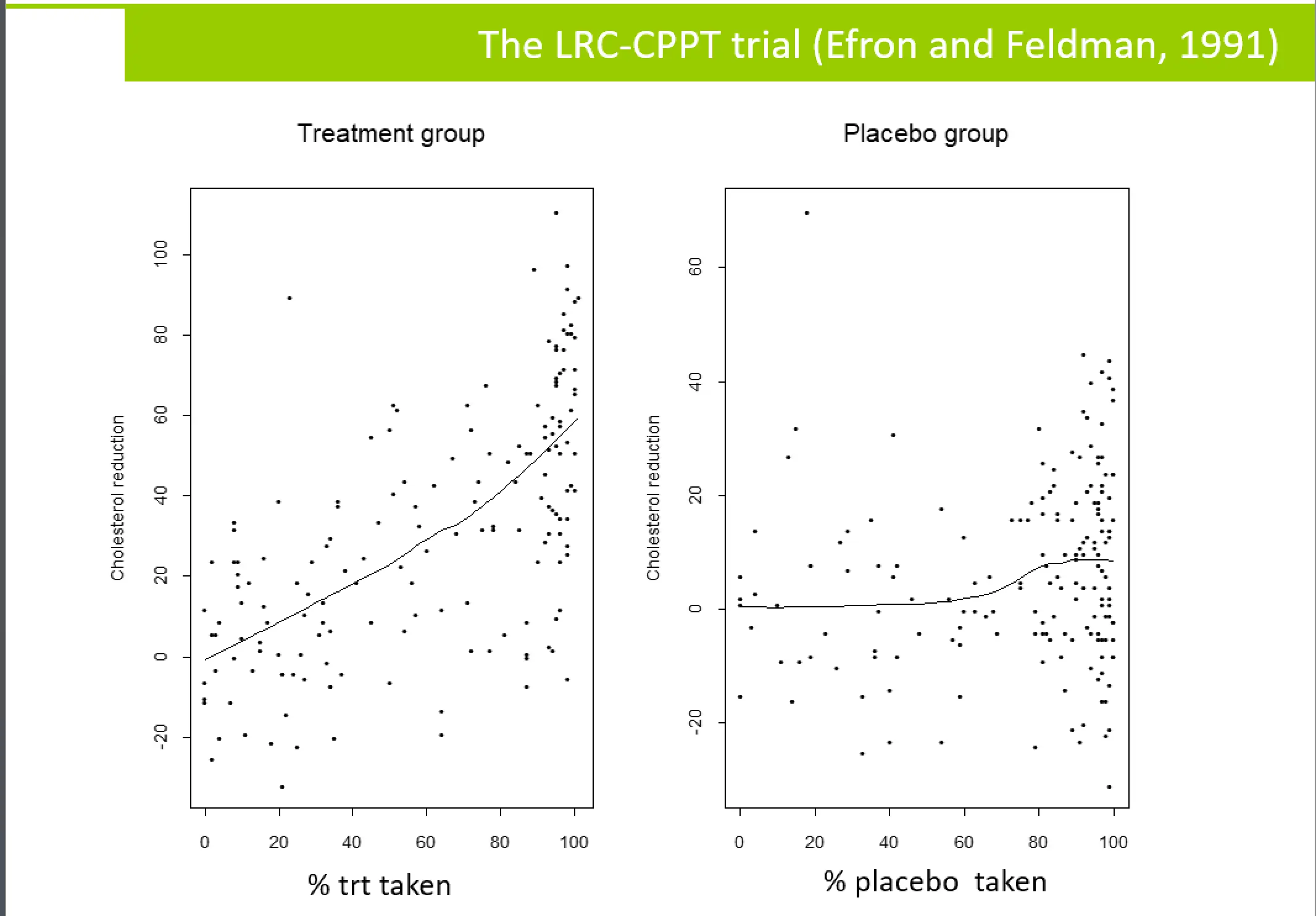
The findings from the CDPRG study have been the subject of a great deal of conjecture and discussion among statisticians and regulators in the intervening years. The first notable instance was a paper by Efron and Feldman, written just over a decade later in 1991, which examined compliance among a portion of patients taking cholestyramine in the Lipid Research Clinics Coronary Primary Prevention Trial. Here again, increased adherence was associated with improved outcomes for both the drug-taking population and control group, albeit with a lower-level response among those taking the placebo.
In 2016, experts in causal inference replicated the CDPRG findings and re-analysed the dataset using updated methods of statistical analysis.4 Their approach reduced the difference in mortality observed among adherers and non-adherers in the placebo group. This led them to conclude that such methods should be used to adjust for post-randomization adherence variability to complement the intention-to-treat (ITT) analysis with a valid estimation of the drug effects according to the prescription specified in the protocol.
Separate research has delved further into the issues surrounding adherence and placebo in clinical trials.5 Drawing on data from the placebo arm of the Lipid Research Clinics Coronary Primary Prevention Trial, the authors investigated the hypothesis that per-protocol effect estimates can be biased because insufficient adjustments are made regarding the prognostic factors that predict adherence. They concluded that adjusting for this bias is made challenging when adherence is measured as a continuous variable, making it important for investigators to consider employing several models in helping to explain the ‘effect’ of adherence to placebo.
In 2006, Simpson et al gathered findings from various studies in an attempt to further understand the relationship between adherence (both to a drug therapy and placebo) and mortality.6 Their meta-analysis of observational studies, published in the BMJ, concluded that there was indeed a link between the two. Furthermore, the authors stated that this link supported the existence of a ‘healthy adherer’ effect, which describes the fact that people who adhere to treatments within trials are more generally likely to observe good adherence habits and to practise healthier lifestyle behaviours.
The existence of the healthy adherer effect is an understandable cause for concern for regulators, since it appears to generate outlying positive results that are unrelated to the efficacy of the drug under investigation. However, regulators have been hesitant to endorse adherence-adjusted analyses as a means of mitigating against this issue, considering it a post-randomization variable. Rather, regulators have looked to address the healthy adherer effect by encouraging medication adherence in drug trials, thus minimizing exposure variability.
Another strategy to cope with placebo response in clinical trials has been to identify and remove strong placebo responders from the trial during a run-in period that takes place after recruitment but prior to randomization.
However, a possible unintended consequence of this strategy is that the resulting pool of study participants will not include the stronger adherers among the wider population. And, since some of these strong adherers would not be included in the randomized study, the trial will potentially miss important insights into treatment effects among those who would have been most compliant with the prescribed protocol. Moreover, the fact that general adherence will be ‘diluted’ in the trial as a result can mean that study power is reduced, and drug effects or harms can be underestimated.
This scenario highlights the fine line that trials must tread when it comes to adopting enrichment strategies. This point is further underlined by industry guidance from the U.S. Food and Drug Administration (FDA), which references various “useful and generally accepted” methods of reducing variability.7 On the one hand, the documentation advocates eliminating patients “who improve spontaneously or have large placebo responses” at the same time that it supports the inclusion of “patients likely to adhere to treatment”.
And while the FDA notes that excluding poor compliers following randomization is not acceptable practice, referencing the link between adherence and outcome (even with placebo) uncovered by the Coronary Drug Project Research Group, there is no mention of the potential shortcomings associated with excluding strong compliers prior to randomization.
One important assumption that underpins all these discussions around adherence is accuracy of measurement. But given that the most common approaches of adherence measurement used in trials are pill count or self-report, the inherent weaknesses of these methods mean there is generally insufficient understanding of true adherence at a granular level, with adherence often standardised into an overall rate.
Employing more advanced methods of measuring adherence, such as digital adherence monitoring, provides the means for trial investigators to support good adherence levels while also addressing the healthy adherer effect. In this scenario, when average study adherence is decreased following the removal of strong placebo responders in a run-in period, the high-accuracy adherence data generated by digital monitoring can be used to inform patient feedback at an individual level. Analysis has shown that this approach encourages adherence and, therefore, maximizes exposure to the trial drug. Indeed, the inclusion of electronically compiled dosing histories within patient interventions delivers an average increase to combined adherence of as much as 19.8%.8
Ultimately, eliciting a more precise measure of adherence using digital monitoring allows for a more reliable and relevant picture of treatment efficacy to emerge, with the temporal sequence between
drug exposure and patient outcome including a deeper understanding of the drug therapy’s relationship to adherence levels. As such, trial investigators and co-ordinators can rely on adherence-informed data with high levels of accuracy and integrity, which can be analysed using validated statistical methods to complement ITT analysis.
In summary, the use of electronic monitoring can be seen as a valuable mechanism for eliciting better data, better analysis and better insights from clinical trials in light of the relationship between placebo and adherence. While it might not add to our understanding of the placebo phenomenon as articulated by T.C. Graves all those years ago, it does provide trial co-ordinators with the means to account for its effect and to ensure study outputs are optimised.
References:
- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2801%2900108-8/fulltext
- https://www.health.harvard.edu/mental-health/the-power-of-the-placebo-effect
- https://www.nejm.org/doi/full/10.1056/NEJM198010303031804
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4942353/
- https://pubmed.ncbi.nlm.nih.gov/32414298/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1488752/
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products
- https://pubmed.ncbi.nlm.nih.gov/23588595/