
The ‘Don’t Miss a Moment’ campaign – another chapter in the story of medication adherence Years from now, in a future where healthcare is far more digitally embedded than it is today, the date of 27 March 2025 might well

Medication adherence packaging for clinical trials is like medication packaging used in clinical practice, but it includes digital connectivity. Hidden sensors inside the packaging capture medication event data in preparation for analysis in centralized adherence software. AARDEX Group's adherence ecosystem includes solutions selected for their ease of use, accuracy, and reliability, creating a network of industry-leading solutions that synchronize with our adherence software, MEMS AS®. Together with our partners, we deliver an approach boasting unparalleled accuracy, helping researchers understand adherence and identify opportunities for risk stratification.


MEDICATION ADHERENCE PACKAGING
Medication adherence packaging features small, hidden microprocessors or sensors that capture and store medication event data. For example, smart blister packs contain trackers in each cavity, activating and capturing the date and time a pill is removed. Smart pill bottles leverage sensors in the cap to track the data when the cap is removed from the medicine container. By capturing data this way, researchers can gather adherence data in a way that provides no interruption to trial participants' daily lives.
Data from the packaging is synchronized with AARDEX Group's real-time medication adherence software, MEMS AS. The software analyzes and processes the data using over seventy pre-defined proprietary algorithms. Adherence intelligence is displayed in sleek, user-friendly dashboard visualizations, highlighting participants and sites with problematic adherence patterns. With MEMS AS, researchers don't have to rely on manual, cumbersome pill counts, reading e-diaries, or urine and blood samples to understand adherence; the software does all the heavy lifting. The payoff? By utilizing this approach, researchers can get to the participants that need coaching, and identify and query adverse events, much sooner than with alternative methods.
Given that poor adherence is prevalent across therapeutic areas and administration routes, we have created an ecosystem of medication adherence packaging solutions covering oral, ophthalmic, topical, and injectable administration. The ecosystem approach delivers a comprehensive yet practical solution for capturing medication event data in clinical trials for all routes of drug administration.

OUR ADHERENCE PACKAGING ECOSYSTEM

MEMS® Cap
Brought to you by AARDEX Group, the MEMS Cap transforms pill bottles into smart pill bottles. With hidden sensors in the cap, dosing events can be captured effortlessly.

Pill Connect
Brought to you by Pill Connect, this smart pill dispenser dispenses the right dose at the right time for participants, reducing the risk of unnecessary medication errors.
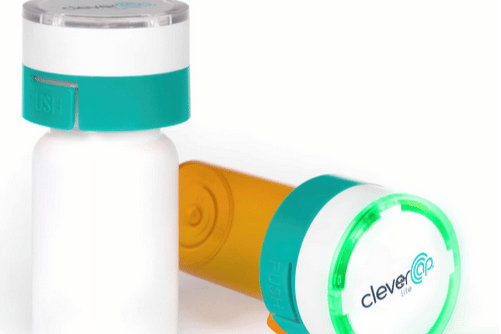
CleverCap Lite®
Brought to you by CMT, the CleverCap digital pill dispenser harnesses light and sound alerts to remind trial participants to take their medications on time.

Dosepak®
Dosepak is an innovative medication compliance packaging solution developed by Westrock. It features a reusable electronic e-module to monitor and record each package opening and closing date and time.

Cerepak®
Cerepak is a patient adherence packaging equipped with hidden processors that accurately capture the date, time, and location when medication is removed from the package.

Smart Blister
brought to you by Schreiner Medipharm, the Smart Bliser features concealed trackers in every cavity that automatically captures the exact time and date when pills or Pre filled syringes are removed

MEMS® HH
Brought to you by AARDEX Group, the Helping Hand automatically captures dosing events when the blister pack is removed from the sleeve.

ID-CapTM
Brought to you by etecRx, the ID-Cap electronic pill system harnesses ingestible sensors to transmit medication event data to a wearable device.

Injectapak®
Brought to you by westrock, Injectapak provides a convenient, easy-to-use solution for measuring adherence to injectable medicines in clinical trials.

MEMS® Button
Brought to you by AARDEX Group, the MEMS Button provides a user-friendly way of understanding how participants engage with the dosing regimen in trials.
Adherence Knowledge Center
Immerse yourself in a wealth of knowledge featuring an abundance of FAQs, case studies, industry expert-led videos, and cutting-edge scientific papers to gain a deeper understanding of medication adherence and why it matters in clinical trials.

Need Help?
Schedule a complimentary adherence consultation with our resident medication adherence expert, Bernard Vrijens.

OUR CLIENTS
Some of the world's leading pharmaceutical companies have embraced our medication adherence packaging. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Medication adherence is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure medication adherence is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Medication adherence packaging brings many benefits. Here are examples:
Real-time monitoring and feedback: can provide real-time monitoring of medication-taking behavior, which can allow healthcare providers to intervene quickly if participants are not taking their medications as prescribed. This can help to prevent adverse events and improve participant outcomes.
Improved adherence: can provide participants with reminders and alerts to take their medications on time, which can improve medication adherence and reduce the risk of medication errors.
Personalized care: can collect data on medication-taking behavior and use this data to personalize participant care, such as adjusting medication regimens based on participant response or providing targeted interventions for participants who are at high risk for non-adherence.
Looking for solutions that cater to inhalable medications? Check out our adherence device solutions here.
There are several types of medication packaging available in clinical trials, each with their own benefits. Here are some examples:
For Oral Dose Drugs
For Injectables:
For Topical Medications:
The benefits of each type of medication packaging may vary depending on the specific requirements of the trial and the participant population being studied. Ultimately, the choice of medication packaging should be based on the goals of the trial, the medication being studied, and the participant population.
Yes – it has been proven to foster better adherence in clinical trials. Digital packaging options have the advantage of being able to passively capture medication event data, which can provide objective and real-time data on medication adherence.
Several studies have evaluated the effectiveness of digital adherence packaging in clinical trials. For example, a randomized controlled trial published in JAMA Internal Medicine in 2016 found that patients using MEMS packaging were more likely to achieve viral suppression in HIV treatment compared to those using standard packaging [1]. Another randomized controlled trial published in the New England Journal of Medicine in 2013 found that patients using MEMS packaging were more likely to adhere to their medication regimen and achieve viral suppression in HIV treatment compared to those using pill counting [2].
The use of digital adherence packaging in clinical trials can also improve the accuracy and reliability of adherence data, which can enhance the validity and generalizability of the study results. Digital packaging can provide researchers with detailed information about medication-taking behavior, including dosing patterns, timing of medication administration, and potential adherence-related issues.
References:
Yes – it has been proven to foster better adherence in clinical trials. Digital packaging options have the advantage of being able to passively capture medication event data, which can provide objective and real-time data on medication adherence.
Several studies have evaluated the effectiveness of digital adherence packaging in clinical trials. For example, a randomized controlled trial published in JAMA Internal Medicine in 2016 found that patients using MEMS packaging were more likely to achieve viral suppression in HIV treatment compared to those using standard packaging [1]. Another randomized controlled trial published in the New England Journal of Medicine in 2013 found that patients using MEMS packaging were more likely to adhere to their medication regimen and achieve viral suppression in HIV treatment compared to those using pill counting [2].
The use of digital adherence packaging in clinical trials can also improve the accuracy and reliability of adherence data, which can enhance the validity and generalizability of the study results. Digital packaging can provide researchers with detailed information about medication-taking behavior, including dosing patterns, timing of medication administration, and potential adherence-related issues.
References:
Yes – it is generally welcomed by trial participants, although acceptance may vary depending on the study population and the specific type of packing play a part. From an acceptability standpoint, selected packaging should be easy to use and not place burden on participants.
One of the reasons why digital packaging is generally well-accepted is that it can provide patients with additional support and tools to help them adhere to their medication regimen. For example, regardless of the packaging or device the participant is using, AARDEX Group’s mobile app, MEMS® Mobile can provide participants with reminders to take their medication, which can be particularly helpful for patients who have difficulty remembering to take their medication on time.
Digital packaging can also provide patients with real-time feedback on their medication-taking behaviors, which can help them stay on track with their medication regimen. This can be particularly helpful for patients who are struggling with adherence-related issues or who are taking complex medication regimens.
In addition, digital packaging can provide patients with a sense of control over their medication regimen. By being able to track their medication-taking behavior and receive feedback on their adherence, patients may feel more empowered and engaged in their healthcare.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.

FROM THE AARDEX GROUP BLOG
We are excited to showcase the expertise of Bernard Vrijens, a globally recognized authority on medication adherence. Non-adherence to medication regimens is a significant challenge that can hinder optimal health outcomes. Leveraging his extensive research and experience, Bernard Vrijens provides invaluable insights into the root causes of medication non-adherence and actionable strategies to address them. Our medication adherence blog features his expert contributions, offering evidence-based recommendations to improve medication adherence and ultimately enhance patient outcomes.

The ‘Don’t Miss a Moment’ campaign – another chapter in the story of medication adherence Years from now, in a future where healthcare is far more digitally embedded than it is today, the date of 27 March 2025 might well

They might not always be easily identifiable, but millions of people across the globe are living with the debilitating symptoms of depression. This common mental disorder is thought to affect around 5% of the world’s adult population, with the pandemic

WHO cares. It’s now time you did Why Change is Needed in Clinical Trials? The old adage tells us not to fix things that aren’t broken. Logic suggests, therefore, that whenever a fix is being applied, things aren’t working as
Enhancing Adherence in Clinical Trials: A Strategic Imperative When a high-profile authority in the medical world, such as the World Health Organization (WHO), publishes guidelines on best practices in clinical trials, the industry takes note. Aiming for Reform and Improvement

Nostalgia can trick us into thinking that things were better in the “good old days”. But the world of science is rarely a place where sentimentality should cloud better judgement. Rather, it is a place where questions, analysis and evidence

When bringing a new drug product to market, powerful motivating forces are involved. And while the potential for enhancing, or even extending, the lives of patients unquestionably takes primacy, there is another inescapably influential factor in the mix: profitability. For