OnDosis Dosage Manager
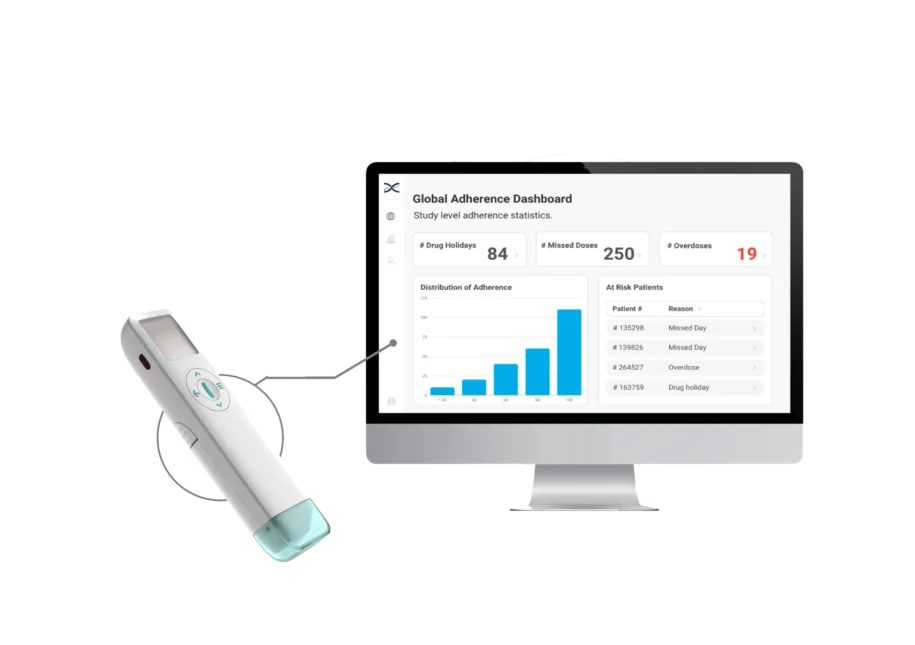
Presented by OnDosis, Dosage Manager is an innovative medication delivery and management solution designed to support patients in clinical trials for oral medications. Its primary goal is to improve adherence by providing an intuitive and patient-friendly dosing system, ensuring that participants take their medication as prescribed. Dosage Manager improves patient outcomes and contributes to more reliable clinical trial results by simplifying the administration process and supporting effective medication management.

OnDosis
How Does the OnDosis Solution Work?
The OnDosis Dosage Manager offers a revolutionary solution by providing precise, individualized dosing of oral medications in solid form, such as granules, pellets, or mini-tablets. This user-friendly device ensures accurate dosing with the click of a button, making it ideal for studies that require strict adherence to personalized dosing regimens.
In clinical trials, where accurate dosing and adherence are critical, the Dosage Manager delivers consistent and reliable doses, minimizing variability in drug administration. Its innovative design supports flexible dosing adjustments, enabling researchers to tailor medication to each participant’s specific needs. This is especially valuable in trials involving personalized or adaptive therapies.
The device integrates seamlessly with AARDEX’s MEMS Adherence Software, enabling researchers and study participants to remotely monitor dosing patterns in real-time. This ensures precise tracking of drug exposure throughout the trial, allowing for timely adjustments to therapy as needed.


FEATURES
FDA-Endorsed Adherence Strategy.
The FDA's guidance, "Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products," directly appeals to the research community to prioritize treatment adherence. In line with this objective, the FDA recommends employing compliance packaging to motivate and encourage participants to follow their treatment regimens. By harnessing technology in treatment management, researchers can adopt a proactive approach, leading to more tailored and efficacious care.
- ICH GCP Compliant
- FDA 21 CFR part 11 Compliant

OUR CLIENTS
The Go-To Solution for Pharma Companies
Some of the world's leading pharmaceutical companies have embraced our medication adherence solutions. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Medication Adherence Packaging →
Discover our range of medication adherence packaging.

Medication Adherence Devices→
Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Frequently Asked Questions
Treatment compliance is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure poor patient compliance is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Treatment compliance, also known as medication adherence, refers to the extent to which a patient follows the prescribed treatment regimen as instructed by their healthcare provider or the clinical trial protocol. This can include taking medications on time, taking the correct dose, and following any specific instructions related to the medication or treatment.
In the context of clinical trials, treatment compliance is essential to ensure the accuracy and validity of the study results. Non-compliance with treatment, such as missing doses or taking incorrect doses, can compromise the study’s accuracy and validity, leading to erroneous or misleading conclusions about the safety and efficacy of the treatment being tested.
Ensuring treatment compliance is essential for patients to maximize the potential benefits of the treatment being tested. For example, in the case of a life-threatening condition, treatment compliance can be the difference between a successful outcome and a negative one. Additionally, medication adherence is crucial for the development and approval of new treatments, as accurate and reliable clinical trial results are required for regulatory approval and widespread use.
Non-compliance in clinical trials refers to a patient’s failure to adhere to the prescribed treatment regimen as directed in the clinical trial protocol. This can include missing doses, taking incorrect doses, or discontinuing treatment altogether.
For patients, non-compliance in clinical trials can have several negative consequences. First, it can compromise the accuracy and validity of the study results, making it more challenging to draw meaningful conclusions about the safety and efficacy of the treatment being tested.
Second, non-compliance may result in diminished treatment efficacy, leading to poorer outcomes for the patient. Third, non-compliance may increase the risk of adverse events, particularly if the treatment requires strict monitoring or has potential side effects.
Additionally, non-compliance can lead to the need for recruiting more study participants, ultimately raising costs and extending trial durations. This can delay the approval and availability of potentially life-changing treatments for patients who are waiting for them.
Overall, non-compliance in clinical trials can significantly impact patients’ health outcomes, delay the availability of new treatments, and increase the cost of developing and testing new medications. It is essential for patients to follow the prescribed treatment regimen carefully to ensure the accuracy and validity of the study results and maximize the potential benefits of the treatment being tested.
When a patient is non-compliant in a clinical trial, it can have various consequences for both the patient and the study itself. The following are some of the potential outcomes:
Reduced study accuracy and validity: Non-compliance can compromise the accuracy and validity of the study results, making it more challenging to draw meaningful conclusions about the safety and efficacy of the treatment being tested. This can delay the approval and availability of potentially life-changing treatments for patients who are waiting for them.
Diminished treatment efficacy: Non-compliance may result in diminished treatment efficacy, leading to poorer outcomes for the patient. This can be particularly harmful if the treatment being tested is meant to treat a life-threatening condition.
Increased risk of adverse events: Non-compliance may increase the risk of adverse events, particularly if the treatment requires strict monitoring or has potential side effects.
Exclusion from the study: In some cases, patients who are consistently non-compliant may be excluded from the study altogether. This can be disappointing for patients who have invested time and effort into the trial.
Increased costs and extended trial durations: Non-compliance may lead to the need for recruiting more study participants, ultimately raising costs and extending trial durations.
Overall, non-compliance in clinical trials can significantly impact the accuracy and validity of the study results, harm patients’ health outcomes, delay the availability of new treatments, and increase the cost of developing and testing new medications. Therefore, it is crucial for patients to follow the prescribed treatment regimen carefully and communicate any difficulties they may be experiencing with their healthcare provider or research team.
Medication event monitoring systems: Medication Event Monitoring Systems, such as the Injectapak and MEMS AS, provides the most precise and accurate assessment of treatment compliance – studies have concluded that this approach is 97% effective and bears minimal burden for patients and sites.
Patient self-reporting: Patients may be asked to self-report their adherence to the treatment regimen through various means, such as daily diaries, questionnaires, or surveys. Self-reporting can provide valuable insights into patient behavior and attitudes towards treatment. However, studies show that this approach adds significant burden for patients and may introduce bias into studies. Further, research has highlighted that the accuracy and reliability of self-reporting to be 27%.
Pill counts: Researchers may conduct periodic pill counts to assess the number of doses taken by patients and compare them to the expected number of doses. This method can provide a quantitative measure of compliance. However, studies have shown that aside from adding significant burden for HCPs, the reliability of the approach comes in at 60%.
Biological markers: Biological markers, such as blood or urine tests, can be used to determine whether patients are taking the medication as prescribed. For example, a low level of medication in the blood or urine may indicate non-compliance. While biological marker sampling provides around a 70% accuracy rate, the data derived from sampling is simply too sparse to understand the evolution of adherence over time.
Direct observation: In some cases, researchers may directly observe patients taking their medication to ensure compliance. This method can be time-consuming and may be difficult to implement in large-scale clinical trials.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.


Collaborating for Safer, More Efficient Trials
By combining technology and partnerships, we are revolutionizing how medication adherence is monitored in clinical trials. Our unique adherence ecosystem brings together leading medication adherence packaging and devices and DCT, IRT, and EDC vendors, CROs, and CMOs to drive innovation.
