NOV-2017 : Incredible, one year has already passed since we presented one of the most interesting projects in medication adherence monitoring. MEMS® (Medication Event Monitoring System) were shown to be a useful tool to increase adherence to PrEP medication in large multi-country projects run in challenging low resource settings.
We walk the talk
The MEMS® (Medication Event Monitoring System) are being used as a tool to measure adherence and guide an intervention in subjects on PrEP medication (Pre-Exposure Prophylaxis for HIV) in a multi-country low resource setting medical practice program.
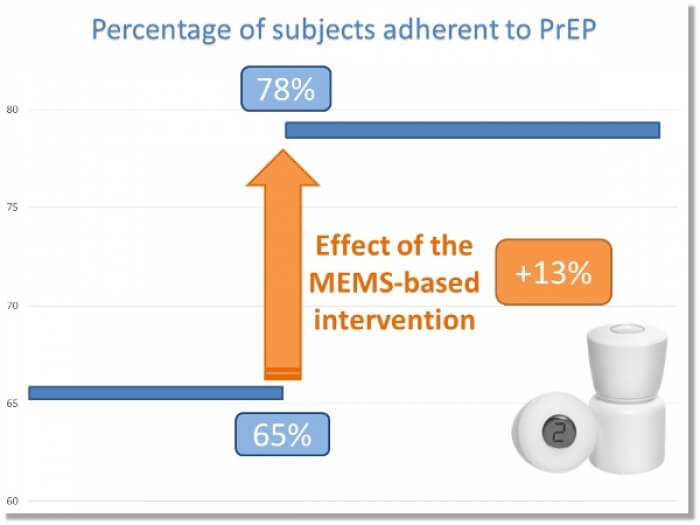
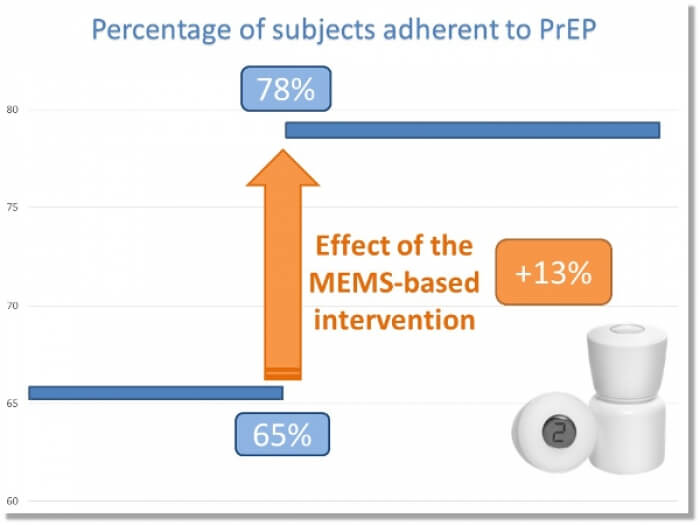
The program is currently initiated in 4 centers located in the vicinity of Dakar, Senegal (N=161) and 5 centers in Nairobi and in remote locations by the Victoria Lake, Kenya (N=786). The dosing history data recorded by the MEMS® is used for individual feedback, at point of care, in a focused discussion between the health service providers and the subjects. After receiving the MEMS® based intervention, the likelihood of taking the medication as prescribed increased by 13% in the targeted population(1).
These preliminary results are encouraging and suggest the feasibility and effectiveness of measurement-guided adherence-enhancing intervention based on MEMS monitoring and feedback in resource-limited settings.
Reach out to us a MEMS@aardexgroup.site to receive more information about the use of the MEMS® (Medication Event Monitoring System).
Best Regards,
The MEMS® team
(1) Espacomp 2016 – Successful measurement-guided Adherence-enhancing intervention in HIV pre-exposure prophylaxis (PrEP) in low-resource settings – Results from interim analysis