Injectapak® Compliance Packaging for Medication
Presented by Westrock, Injectapak is a specialized compliance packaging for medications designed to support participants in clinical trials investigating injectable medicines. Its primary goal is to reduce non-compliance with treatment by providing a convenient and participant-friendly reminder system, ensuring participants administer their injections as prescribed. Injectapak improves participant outcomes and ensures more accurate clinical trial results by simplifying the process and assisting with medication management.

Injectapak
Trusted Solution for Identifying Non-Compliance
Identifying non-compliance with treatment is an essential factor in conducting clinical trials. Failure to comply, particularly with injectable medications, can have far-reaching consequences, including compromised study accuracy, diminished treatment efficacy, increased risk of adverse events, and extended trial durations, which can raise research costs significantly.
User-friendly Compliance Packaging for Medications
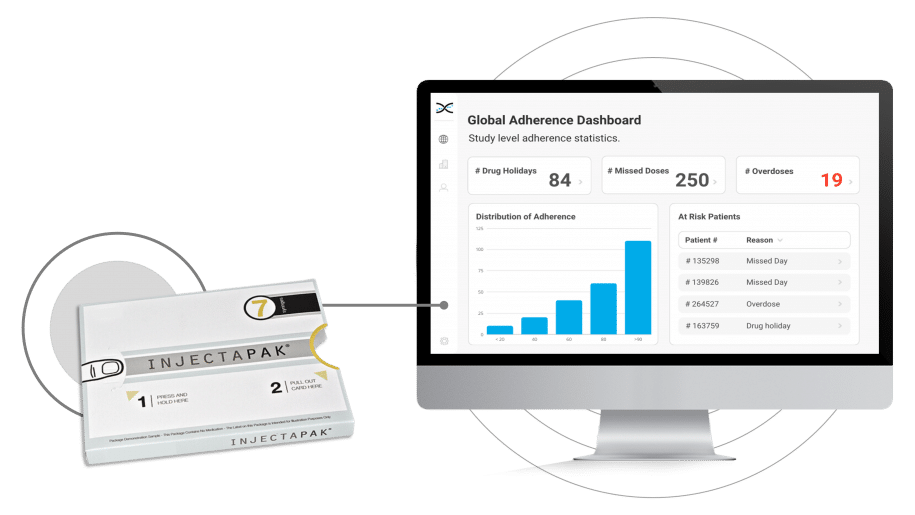
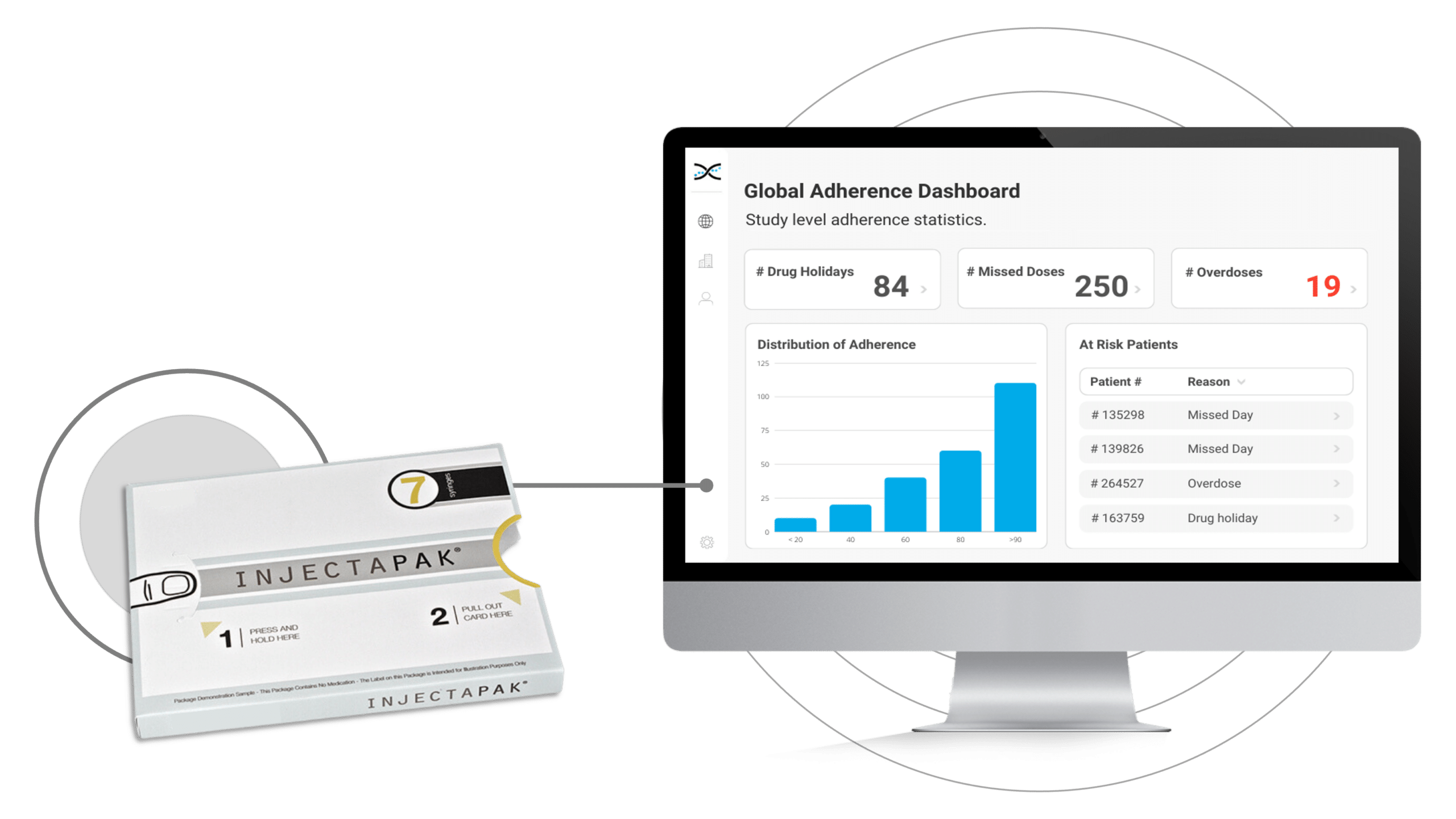
To overcome these challenges, the pharmaceutical industry has embraced Westrock's Injectapak, a user-friendly compliance packaging for medications. Injectapak enhances compliance by providing precise and straightforward dosing instructions for participants, reducing the likelihood of self-administration errors, and improving treatment adherence. By addressing poor compliance with treatment early on, clinical trials can proceed more efficiently, yielding more precise results and ultimately leading to enhanced participant treatment outcomes.Moreover, Injectapak is fully compatible with AARDEX Group's treatment adherence software, MEMS AS®. The integration of these two cutting-edge technologies enables real-time, remote compliance monitoring for clinical researchers. By syncing data gathered by Injectapak with MEMS AS, compliance dashboards and reports generate automatically, offering insights into treatment usage trends. This crucial information can help identify participants struggling with adherence and facilitate adjustments to treatment plans as necessary.
The use of Injectapak offers a comprehensive solution to the challenges of non-compliance. By improving compliance, Injectapak and MEMS AS can lead to more reliable and precise results, benefiting researchers and patients alike.
Features of the Injectapak® Compliance Packaging Integrated with MEMS AS®

FEATURES
Detect Non-Adherence in Real-Time.
In conjunction with AARDEX Group's treatment adherence software, MEMS AS, Injectapak serves as a formidable solution for identifying non-compliance with treatment in clinical trials. Its sophisticated microprocessor technology enables precise treatment usage data collection. MEMS AS processes the data using more than seventy proprietary algorithms to create dashboard visualizations and reports on compliance at various levels, including participant, study, and site. This fusion of technologies establishes a new benchmark in medication compliance monitoring. It equips investigators with unparalleled insights into how participants interact with treatments, empowering them to make well-informed decisions and implement measures that enhance participant outcomes.
- Fully Scalable
- ISO27001 Certified Datacenter
- HIPAA & GDPR Compliant
- IRT, EDC & DCT Integration

FEATURES
Medication Reminders with MEMS® Mobile.
Overcoming obstacles to treatment compliance in clinical trials is essential for enhancing participant outcomes and preserving research integrity. We understand the significance of providing user-friendly solutions that impose minimal burden on participants, requiring only that they follow their prescribed treatment regimen Injectapak integrates seamlessly with MEMS® Mobile, a handy app that enables participants to schedule medication reminders, assisting them in adhering to their dosing schedules. By adopting a participant-focused approach to treatment compliance and integrating Injectapak with MEMS Mobile, we aim to empower participants and support them in achieving improved health outcomes. Our commitment to this approach ensures that participants and researchers benefit from reliable and accurate clinical trial results.
- User-Friendly App
- IoS & Android Compatible
- Available in 28 Languages
- 20K+ Users in 30 Countries
FEATURES
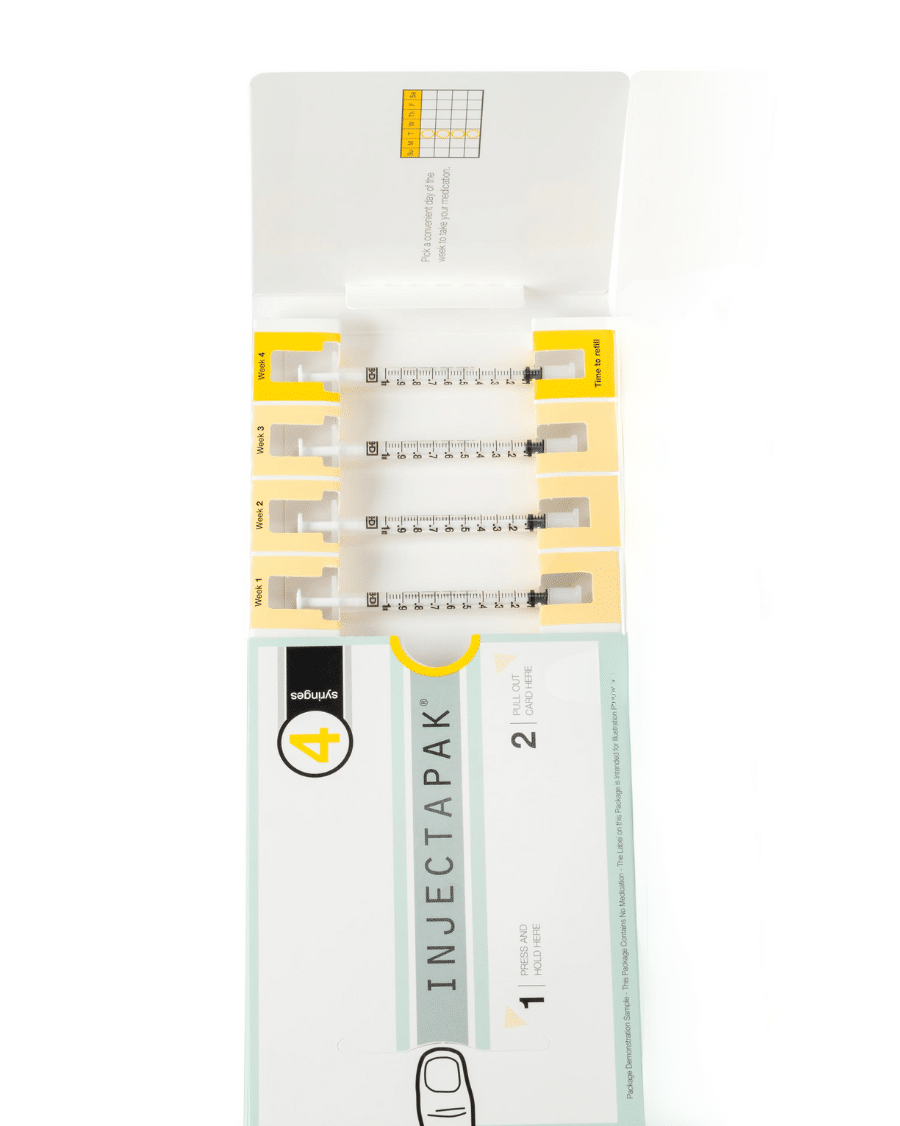
Flexible Compliance Packaging for Injectables.
Injectapak offers weekly or monthly dosing packages, simplifying injection management for participants, even when faced with complex treatment schedules. By streamlining the injection management process, Injectapak significantly enhances compliance with treatment, ensuring that participants administer the right doses at the right time intervals. Equipped with a user-friendly design, flexible dosing options, and a focus on participant convenience, Injectapak is raising the bar in compliance packaging. This innovative approach to adherence helps researchers and healthcare providers better understand the effectiveness of medications and supports the development of more effective treatment plans.
- Tamper-evident Design
- Durable construction
- Child-resistant
- Patient-Centric Design
- Suitable for Vials and Syringes

FEATURES
FDA-Endorsed Adherence Strategy.
The FDA's guidance, "Enrichment Strategies for Clinical Trials to Support Determination of Effectiveness of Human Drugs and Biological Products," directly appeals to the research community to prioritize treatment adherence. In line with this objective, the FDA recommends employing compliance packaging to motivate and encourage participants to follow their treatment regimens. By harnessing technology in treatment management, researchers can adopt a proactive approach, leading to more tailored and efficacious care.
- ICH GCP Compliant
- FDA 21 CFR part 11 Compliant

OUR CLIENTS
The Go-To Solution for Pharma Companies
Some of the world's leading pharmaceutical companies have embraced our medication adherence solutions. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Medication Adherence Packaging →
Discover our range of medication adherence packaging.

Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Frequently Asked Questions
Treatment compliance is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure poor patient compliance is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Treatment compliance, also known as medication adherence, refers to the extent to which a patient follows the prescribed treatment regimen as instructed by their healthcare provider or the clinical trial protocol. This can include taking medications on time, taking the correct dose, and following any specific instructions related to the medication or treatment.
In the context of clinical trials, treatment compliance is essential to ensure the accuracy and validity of the study results. Non-compliance with treatment, such as missing doses or taking incorrect doses, can compromise the study’s accuracy and validity, leading to erroneous or misleading conclusions about the safety and efficacy of the treatment being tested.
Ensuring treatment compliance is essential for patients to maximize the potential benefits of the treatment being tested. For example, in the case of a life-threatening condition, treatment compliance can be the difference between a successful outcome and a negative one. Additionally, medication adherence is crucial for the development and approval of new treatments, as accurate and reliable clinical trial results are required for regulatory approval and widespread use.
Non-compliance in clinical trials refers to a patient’s failure to adhere to the prescribed treatment regimen as directed in the clinical trial protocol. This can include missing doses, taking incorrect doses, or discontinuing treatment altogether.
For patients, non-compliance in clinical trials can have several negative consequences. First, it can compromise the accuracy and validity of the study results, making it more challenging to draw meaningful conclusions about the safety and efficacy of the treatment being tested.
Second, non-compliance may result in diminished treatment efficacy, leading to poorer outcomes for the patient. Third, non-compliance may increase the risk of adverse events, particularly if the treatment requires strict monitoring or has potential side effects.
Additionally, non-compliance can lead to the need for recruiting more study participants, ultimately raising costs and extending trial durations. This can delay the approval and availability of potentially life-changing treatments for patients who are waiting for them.
Overall, non-compliance in clinical trials can significantly impact patients’ health outcomes, delay the availability of new treatments, and increase the cost of developing and testing new medications. It is essential for patients to follow the prescribed treatment regimen carefully to ensure the accuracy and validity of the study results and maximize the potential benefits of the treatment being tested.
When a patient is non-compliant in a clinical trial, it can have various consequences for both the patient and the study itself. The following are some of the potential outcomes:
Reduced study accuracy and validity: Non-compliance can compromise the accuracy and validity of the study results, making it more challenging to draw meaningful conclusions about the safety and efficacy of the treatment being tested. This can delay the approval and availability of potentially life-changing treatments for patients who are waiting for them.
Diminished treatment efficacy: Non-compliance may result in diminished treatment efficacy, leading to poorer outcomes for the patient. This can be particularly harmful if the treatment being tested is meant to treat a life-threatening condition.
Increased risk of adverse events: Non-compliance may increase the risk of adverse events, particularly if the treatment requires strict monitoring or has potential side effects.
Exclusion from the study: In some cases, patients who are consistently non-compliant may be excluded from the study altogether. This can be disappointing for patients who have invested time and effort into the trial.
Increased costs and extended trial durations: Non-compliance may lead to the need for recruiting more study participants, ultimately raising costs and extending trial durations.
Overall, non-compliance in clinical trials can significantly impact the accuracy and validity of the study results, harm patients’ health outcomes, delay the availability of new treatments, and increase the cost of developing and testing new medications. Therefore, it is crucial for patients to follow the prescribed treatment regimen carefully and communicate any difficulties they may be experiencing with their healthcare provider or research team.
Medication event monitoring systems: Medication Event Monitoring Systems, such as the Injectapak and MEMS AS, provides the most precise and accurate assessment of treatment compliance – studies have concluded that this approach is 97% effective and bears minimal burden for patients and sites.
Patient self-reporting: Patients may be asked to self-report their adherence to the treatment regimen through various means, such as daily diaries, questionnaires, or surveys. Self-reporting can provide valuable insights into patient behavior and attitudes towards treatment. However, studies show that this approach adds significant burden for patients and may introduce bias into studies. Further, research has highlighted that the accuracy and reliability of self-reporting to be 27%.
Pill counts: Researchers may conduct periodic pill counts to assess the number of doses taken by patients and compare them to the expected number of doses. This method can provide a quantitative measure of compliance. However, studies have shown that aside from adding significant burden for HCPs, the reliability of the approach comes in at 60%.
Biological markers: Biological markers, such as blood or urine tests, can be used to determine whether patients are taking the medication as prescribed. For example, a low level of medication in the blood or urine may indicate non-compliance. While biological marker sampling provides around a 70% accuracy rate, the data derived from sampling is simply too sparse to understand the evolution of adherence over time.
Direct observation: In some cases, researchers may directly observe patients taking their medication to ensure compliance. This method can be time-consuming and may be difficult to implement in large-scale clinical trials.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.


Collaborating for Safer, More Efficient Trials.
By combining technology and partnerships, we are revolutionizing how medication adherence is monitored in clinical trials. Our unique adherence ecosystem brings together leading medication adherence packaging and devices and DCT, IRT, and EDC vendors, CROs, and CMOs to drive innovation.
