ID-CapTM Electronic Pill
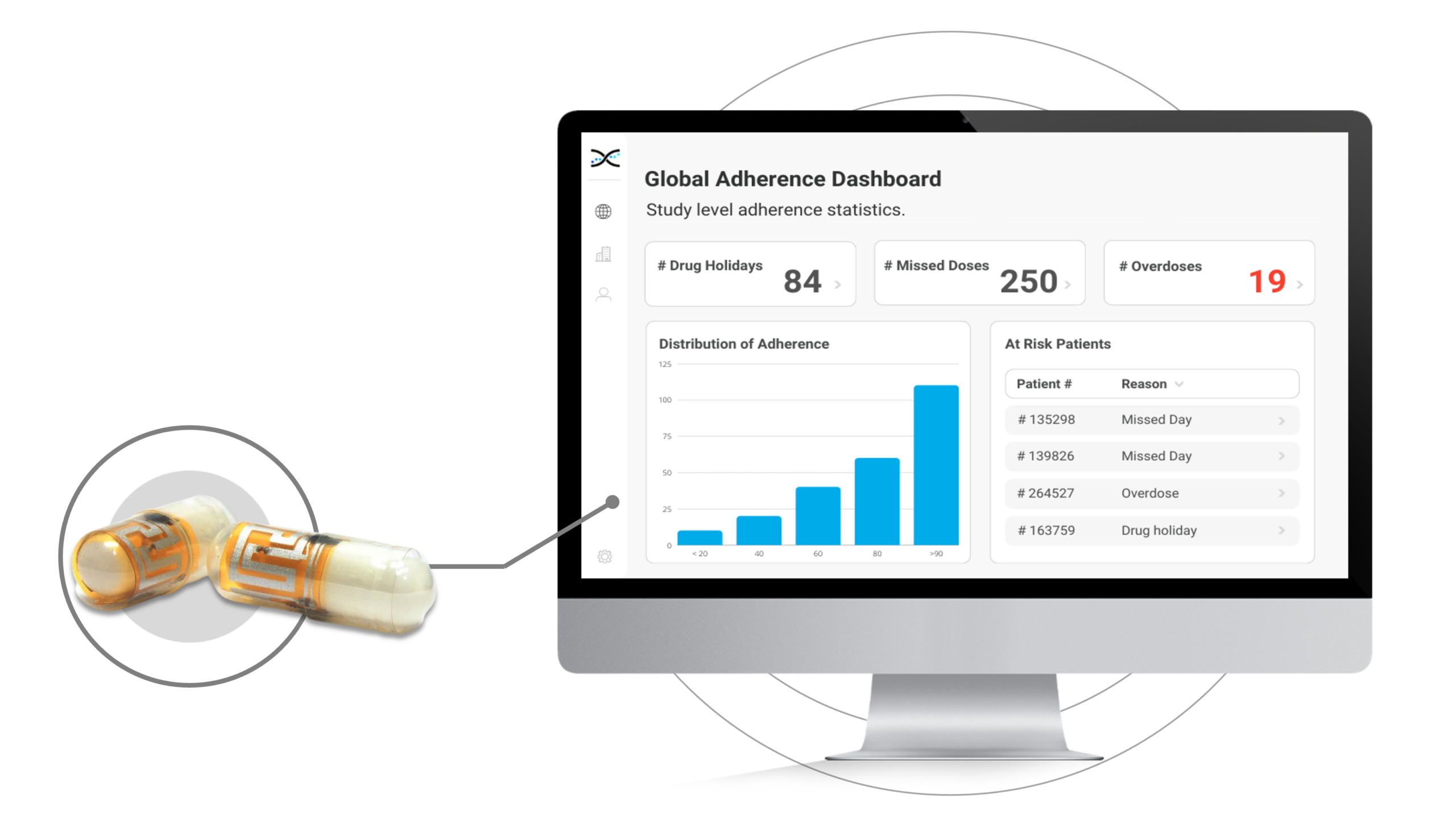
The ID-Cap is no ordinary pill. It's an electronic pill that packs an ingestible wireless sensor called the ID-TagTM. When ingested, the ID-Tag taps into etectRx's unique communication technology to beam a low-power digital signal right from the patient's stomach, automatically transmitting the date and time of ingestion to a wearable device. The data collected by the electronic pill can be conveniently accessed from within AARDEX Group's Medication Adherence Software, MEMS AS®. MEMS AS is a cloud-based software with easy-to-use dashboards and real-time adherence insights.

ID-CAP ELeCTRONIC Pill
The Electronic Pill, A Practical Solution to
an Age-Old Problem
Electronic Pills have the potential to address an age-old problem, poor adherence in clinical trials. Adherence refers to the extent to which patients take their medication as prescribed, and it plays a vital role in determining the safety and efficacy of drugs. If patients don't take their medications as prescribed, it can result in increased variability and reduced statistical power, ultimately jeopardizing researchers' ability to answer the research question.
Together with adherence software, electronic pills offer a practical solution for understanding how patients interact with their prescribed dosing regimen. Unlike alternative methods, which can be time-consuming for patients and research staff alike, the electronic pill captures medication intake data passively, without any intervention.
How does the electronic pill work?
The etectRx electronic pill features a capsule with an embedded ingestible wireless sensor called the ID-Tag. When swallowed, the sensor within the electronic pill transmits a low-power digital message from inside the stomach, transmitting the date and time of ingestion to a wearable device that detects messages sent from ingested ID-Tags and forwards them using Bluetooth technology to the ID-Cap App on the patient's smartphone.Features of the ID-CapTM Electronic Pill Integrated with MEMS AS®

FEATURES
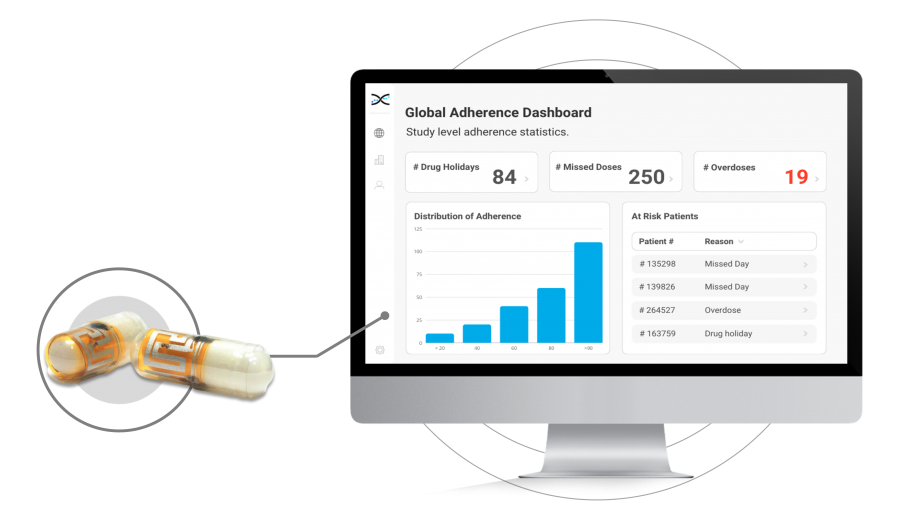
Electronic Pills Provide Real-Time Data.
The ID-Cap electronic pill integrates with AARDEX Group’s adherence Software, MEMS AS, providing a comprehensive solution for researchers to monitor adherence to oral dose medications. The cloud-based software includes pre-built dashboards and lists that provide researchers with a deep understanding of how patients are taking their medicines. With these adherence insights readily available, researchers can promptly identify adherence issues and provide coaching and support to patients
- Fully Scalable
- ISO27001 Certified Datacenter
- HIPAA & GDPR Compliant
- IRT, EDC & DCT Integration

FEATURES
FDA Approved Electronic Pill.
etectRx’s electronic pill has gone through FDA’s 510(k) clearance process, which earned it approval for use in 2019. The process involves a rigorous review of the device's design, performance, and clinical data to ensure that it meets the necessary safety and effectiveness standards. Gaining FDA clearance reinforces etectRx’s unwavering commitment to providing a practical, safe, and effective solution for measuring adherence to prescribed medications in clinical trials. Since gaining FDA clearance, the technology has surpassed 10,000 ingestions and has generated strong, accurate results across multiple indications, particularly with patient populations with traditionally poor levels of adherence, including those with substance abuse disorders.
- Self-Powered by Stomach Fluids
- Looks, Tastes, and Feels Just Like an Ordinary Capsule

FEATURES
Electronic Pills Boost Engagement.
Boasting an awe-inspiring 98% user engagement rate, The ID-Cap electronic pill is no ordinary solution. It's a high-impact solution that skyrockets patient engagement and dramatically boosts the odds of study success. An 8-month acceptability study recently revealed that patients used the ID-Cap on 1245 out of 1270 use days, and there was no sign of dwindling patient engagement during the course of the study. The ID-Cap's integration with MEMS AS unleashes a whole new world of possibilities for researchers to elevate study outcomes and forge meaningful connections with patients that were once deemed unattainable.
- Patient-Friendly
- Easy-to-Use

FEATURES
Companion App
Eliminating barriers to medication adherence in clinical trials is paramount for optimizing participant outcomes and maintaining research integrity. We recognize the importance of user-friendly solutions that place no burden on participants, simply requiring them to take their medication as prescribed. In addition, our solutions offer participants access to MEMS® Mobile, a convenient app that allows them to schedule medication reminders, helping them to stay on top of their dosing regimen. Our patient-centric approach to medication adherence strives to empower participants and help them achieve better outcomes.
- User-Friendly App
- IoS & Android Compatible
- Available in 25 Languages
- 20K+ Users in 30 Countries

OUR CLIENTS
The Go-To Solution for Pharma Companies
Some of the world's leading pharmaceutical companies have embraced our medication adherence solutions. From global giants to niche players, these organizations have recognized the value of our innovative solutions for enhancing medication adherence, reducing costs, and improving patient outcomes. It's an honor to partner with these remarkable brands, and we're proud to contribute to their efforts in advancing healthcare.
Medication Adherence Software →
Learn about our industry-leading adherence software for trials.

Medication Adherence Packaging →
Discover our range of medication adherence packaging.

Discover our range of medication adherence devices.
Got Questions?
Connect with an adherence expert.
Frequently Asked Questions
Adherence is a vital yet often overlooked aspect of successful research. That's why we've gone the extra mile to gather and organize the most frequently asked questions about this critical topic. Our goal is to empower researchers and patients alike with the knowledge they need to ensure medication adherence is never a hurdle to progress. So, without further ado, here are the answers you've been looking for!
Electronic pills are made of a combination of materials that are safe for ingestion and compatible with the body. The outer shell of the electronic pill is typically made of an inert material, such as gelatin, that is safe for consumption and can dissolve in stomach acid. Inside the pill, there is a small sensor or microchip that is encased in a protective material, such as a biocompatible polymer, that can withstand the acidic environment of the stomach. The sensor or microchip inside the pill is typically made of materials such as silicon, magnesium, or copper that can transmit data wirelessly to an external device.
Yes. The etectRx electronic pill has gone through rigorous testing and achieved FDA 510(K) status in 2019. Since its approval, it has has surpassed 10,000 ingestions and has generated strong, accurate results across multiple indications, particularly with patient populations with traditionally poor levels of adherence, including those with substance abuse disorders.
WEBINAR WITH MERCK & BIOGEN
Mitigating the Risk of Poor Adherence in Trials
Watch this live recording with adherence experts from Merck & Biogen to learn about their approach to mitigating the risk of poor adherence in trials.


Collaborating for Safer, More Efficient Trials.
By combining technology and partnerships, we are revolutionizing how medication adherence is monitored in clinical trials. Our unique adherence ecosystem brings together leading medication adherence packaging and devices and DCT, IRT, and EDC vendors, CROs, and CMOs to drive innovation.
