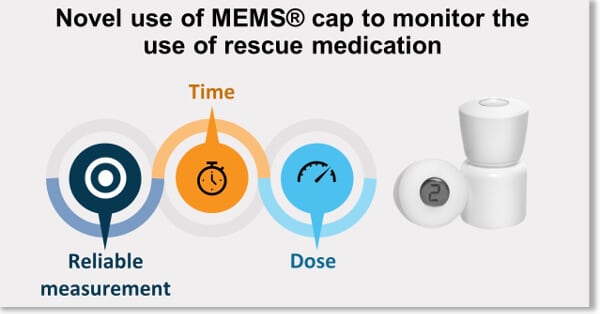
DEC-2017 : MEMS® was used successfully to show the adequate coverage of a new once daily pain treatment in the presence of rescue medication in a Phase III Clinical Trial.
In the Study of Meloxicam Capsules to Treat Osteoarthritis Pain, a Phase III trial designed to evaluate the efficacy and safety of Meloxicam Capsules, the MEMS® cap was used to show the adequate coverage of a new once daily treatment in the presence of rescue medication for the osteoarthritic pain of the knee or hip.
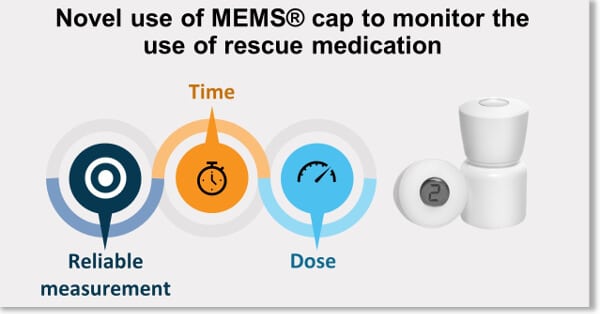
This study addressed the concerns that the once daily dosing regimen might not provide enough coverage for the 24-hour period and that patients might need additional pain medication before it was time for them to take their next daily dose. By using the MEMS® to monitor the use and time of the rescue medication the data showed that patients receiving VIVLODEX in the 5 mg and 10 mg doses used significantly less rescue medication over the course of the study, took fewer daily doses of rescue medication, and for fewer days compared with patients receiving the placebo. Regardless of the time of day, patients treated with VIVLODEX took about half as many rescue pain medication doses as patients receiving the placebo (1).
This study is an example of the successful implementation of the MEMS® in clinical trial to monitor the use of medication.
Reach out to us at MEMS@aardexgroup.site to receive more information about the MEMS® ecosystem.