
As humans, the unconscious act of making assumptions is inherent in our DNA. These mental shortcuts are deep wired into our brains as a way of quickly making sense of the world around us. And yet, throughout history, great thinkers

In a world where clinical trials can make or break the success of a new medication, there's one critical factor that can't be overlooked: medication adherence. That's where our blog comes in. We're here to provide you with the latest industry news, practical tips, and expert advice on everything related to medication adherence in trials.

Get our monthly newsletter delivered to your inbox.

As humans, the unconscious act of making assumptions is inherent in our DNA. These mental shortcuts are deep wired into our brains as a way of quickly making sense of the world around us. And yet, throughout history, great thinkers

The perfectionists among us might outwardly disagree, but, in truth, even they would secretly admit that it is not possible to live a life free from mistakes. We are complex beings living and working in complex environments and, therefore, we
Clinical trials rely on accurate data. But what if one of the most important variables whether a patient actually takes the medication is based on guesswork? Traditional methods like pill counts, diaries, or verbal confirmation have long been the standard,

Imagine spending years developing a promising new treatment, only to have its potential clouded by something as simple (and as overlooked) as whether or not people actually took their pills. This isn’t just a small glitch in the process. In
You’ll often hear the terms “adherence” and “compliance” used as if they mean the same thing. But in healthcare and clinical research, they carry very different meanings. Understanding that difference can change the way we work with patients, design better
Liège, Belgium and Manchester, UK; August 2025: AARDEX Group, a global leader in digital medication adherence solutions, and Pill Connect, a pioneer in connected dispensing technology, have announced a strategic partnership to support a multi-cohort Phase 2A dose selection trial.

Medication adherence is an element of successful clinical research. It refers to the degree to which a patient follows a prescribed medication regimen including when and how often the medication is taken, and whether it is discontinued prematurely. In clinical

Back in 1981 Zanussi, the Italian manufacturer of home appliances, launched one of the most iconic marketing slogans ever – ‘The appliance of science’. Aiming to convey the message that Zanussi appliances were built with a high level of engineering

The Importance of Protocol Design in Drug Development In the early stages of drug development, as the promise surrounding a new therapy builds, there is a palpable sense of the stakes being raised. And when encouraging findings lead to progress

The ‘Don’t Miss a Moment’ campaign – another chapter in the story of medication adherence Years from now, in a future where healthcare is far more digitally embedded than it is today, the date of 27 March 2025 might well

Understanding Depression and Its Impact They might not always be easily identifiable, but millions of people across the globe are living with the debilitating symptoms of depression. This common mental disorder is thought to affect around 5% of the world’s

WHO cares. It’s now time you did Why Change is Needed in Clinical Trials? The old adage tells us not to fix things that aren’t broken. Logic suggests, therefore, that whenever a fix is being applied, things aren’t working as
Enhancing Adherence in Clinical Trials: A Strategic Imperative When a high-profile authority in the medical world, such as the World Health Organization (WHO), publishes guidelines on best practices in clinical trials, the industry takes note. Aiming for Reform and Improvement

Introduction: The Role of Innovation in Clinical Trials Nostalgia can trick us into thinking that things were better in the “good old days”. But the world of science is rarely a place where sentimentality should cloud better judgement. Rather, it

When bringing a new drug product to market, powerful motivating forces are involved. And while the potential for enhancing, or even extending, the lives of patients unquestionably takes primacy, there is another inescapably influential factor in the mix: profitability. For
The economic model that has underpinned the development of drug products for decades is currently under siege. Faced on one side with mounting pressure from payers to address affordability issues, pharmaceutical companies are facing ever more stringent regulatory requirements alongside

Liège, Belgium and Gothenburg, Sweden – October 15, 2024: AARDEX Group, a leader in managing drug exposure in clinical trials, and precision medicine innovators, and OnDosis, a game changer in precision dosing of oral solid medicines through the novel Dosage

The Rise of GLP-1 Therapies In recent years, the pharmaceutical industry has borne witness to the meteoric rise of glucagon-like peptide-1 receptor agonist (GLP-1) therapies. Having fast become established as a potentially life-changing treatment for Type 2 diabetes and obesity, emerging

Global Cancer Challenges and the Need for Effective Dose Optimisation Let us first recognise the enormity of the challenges we are facing, on a global scale, when it comes to cancer. It is estimated that there were 18.1 million new

AARDEX Group, the global leader in medication adherence solutions, has today announced a collaboration agreement with Compliance Meds Technologies (CMT), a mobile-health technology solutions provider. AARDEX MEMS® Adherence Software with Compliance Meds Technologies CleverCap® In this cooperation, the partners will
Medication adherence is a vital component in managing chronic diseases effectively. However, it remains a significant challenge, with non-adherence rates reaching up to 50% among patients. This lack of adherence results in poor health outcomes and increased burdens on healthcare

In a recent paper titled ‘The impacts of undetected nonadherence in phase II, III and post-marketing clinical trials: An overview’ the authors detailed the findings of a research project which aimed to provide an overview of the consequences of unrecognized

Clearly it is in no one’s interests for a clinical trial to fail. Whenever these high-stake activites are abandoned and drug development pathways are aborted, missed opportunities abound. Beyond the sponsor’s missed commercial opportunity, there are missed opportunities to advance

In a clinical trial, every piece of data contributes to the overall story being told. No matter how small or seemingly insignificant, all the information that emerges over the course of the process adds rich detail to the narrative. Outcome

AARDEX Group Belgium – SHL Medical, a world-leading provider of drug delivery devices, and AARDEX Group, the global leader in medication adherence solutions, today announced their strategic partnership aimed at delivering an end-to-end solution for pharma customers seeking to demonstrate

It is perhaps ironic that Alexander Graham Bell, often singled out as the individual who invented the telephone, was a man who believed in the value of collective endeavour. Indeed, Bell is quoted as saying: “Great discoveries and improvements invariably

In 1920, The Lancet published a paper authored by T.C. Graves entitled ‘Commentary on a case of hystero-epilepsy with delayed puberty: Treated with testicular extract’. While the article was not the first to mention the placebo effect, it is recognised

Recognizing the problem: Status quo bias in adherence measurement Among the many forces that can influence clinical trials, arguably not enough attention is paid to status quo bias. A common phenomenon, status quo bias can explain why any proposed departure

In observance of Rare Disease Day, it is imperative to underscore the progressive strides within the domain of rare disease research. Rare diseases, characterized by their infrequent occurrence within the general populace, impose significant burdens not only on the affected

Familiar methods, familiar risks It is human nature for people to trust the things that are known to them ahead of the things that are not. Familiarity, after all, brings with it a sense of comfort and reassurance. It is

In this article, AARDEX Group Quality Manager, Oana Paun, discusses ALCOA+ and its importance for achieving regulatory compliance. Not all data is created equal For those with a scientific ear, it can be reassuring when you hear ideas discussed in

The list of Neurological conditions is considerable, with epilepsy, Alzheimer’s disease, dementia, stroke, migraine, multiple sclerosis, Parkinson’s disease, neuro infections, and brain tumors, all attributed to this category of disease. Hundreds of millions of people worldwide are affected by neurological

It’s an uncomfortable truth that decision-making is a murky business. While we would all like to think that our actions and the choices underpinning them are rooted in logic, integrity, and reason, the truth is not entirely clear-cut. In reality,

In this article, Dr. Vrijens shares his thoughts on the need for dose optimization in the interests of balancing safety and efficacy. How we use language has a huge bearing on the meaning we convey. Take the word ‘overdose’, for

In this article, Dr. Bernard Vrijens discusses the importance of medication adherence data in clinical trials and why medication adherence should be a calculation, not an estimation. Why Medication Adherence Data Matters in Clinical Trials The world is today collecting

In this article, our Scientific Lead, Bernard Vrijens, discusses the influence of medication non-adherence on ROI in clinical trials. Get ready for an insightful read! Fraught with risk yet pivotal to the future success of a drug candidate, clinical trials

This action, if finalized, will require applicants to create a new type of medication guide, referred to as Patient Medication Information (PMI). In this article, our Scientific Lead, Bernard Vrijens, unpacks the proposals and questions whether more is needed to

Adherence specialists join multi-stakeholder CancerX initiative to improve long-term pathways for cancer patients and meet the challenge of oral treatments. Liege, Belgium- July 18, 2023: Adherence specialist AARDEX Group is proud to announce it is a founding member of the CancerX initiative, bringing

In recent decades we have witnessed a shift in how chemotherapy drugs are administered. Historically, cytotoxic chemotherapies were almost exclusively administered by a healthcare professional (HCP) via injection or intravenous drip, but today, oral chemotherapy drug forms are becoming increasingly

Integration of AARDEX MEMS AS and MEMS Mobile technology with Mevia’s Medose dispenser offers opportunity to pilot next stage in reusable and real-time adherence solutions. Liege, Belgium- June 8th, 2023: Adherence specialist AARDEX Group is proud to announce its partnership with Mevia, a

Extending the Verification, Analytical Validation and Clinical Validation (V3+) Framework Project will ensure digital clinical measures and products meet the needs of larger audiences. Liege, Belgium- May 3, 2023: Adherence specialists AARDEX Group is proud to announce its partnership with other industry leaders

Exciting news! AARDEX Group is gearing up for one of the most highly anticipated events in the clinical trials industry – the 13th Annual Outsourcing in Clinical Trials Europe conference, which will take place in the beautiful city of Barcelona

In this article, our Scientific Lead and Adherence Expert, Bernard Vrijens, explores the use of pill count compliance in clinical trials and its reliability for measuring medication adherence. Pressure is ubiquitous when it comes to conducting clinical trials. Sponsors feel the

Dr. Job van Boven, Associate Professor at the University Medical Center Groningen, shares his invaluable insights on the evolution of medication adherence monitoring solutions. According to Dr. Job van Boven, Associate Professor at the University Medical Center Groningen, Founding Director of

About the CMO Step-Up Challenge What is the #CMOStepUp Challenge all about, you may ask? It’s a step challenge where participants will compete to clock the most steps and emerge victorious with the Meta Quest VR Headset as their reward.

Greetings and salutations! It’s time for the March issue of the Medication Monitoring Matters newsletter, where we delve into cutting-edge advancements in medication adherence monitoring. In this edition, we’ll examine the potential downsides of Video Directly Observed Therapy (VDOT) as

The Role of Medication Adherence in Drug Trials Drug trials are conducted to evaluate the safety and efficacy of new or investigational drugs. The primary goal of drug trials is to determine if a new drug is effective in treating

In clinical trial analysis, it’s assumed that participants are following the instructions for the investigational product. Unfortunately, the evidence suggests that this is far from reality. Recently, Kenneth Getz, Executive Director and Professor at the Tufts Center for the Study

“Objective and granular” digital adherence measures could improve trial efficiency and allow for better-informed decisions on the efficacy and safety of new drug products. That’s according to the authors of a literature review that looked at more than 100 medicines

Greetings from the forefront of innovation in clinical trials. We’re thrilled to introduce you to the first edition of our medication adherence newsletter, Medication Monitoring Matters. Our focus? Empowering clinical trial sponsors like you to elevate the standard of care

With the current push for increased diversity in research, the medical community has accepted the challenge to make trial populations more representative of those in clinical practice. Yet poor medication adherence, which clouds the view of how drugs work in

Seamless integration of adherence and research data protects participant safety and data quality, with no additional burden for clinical trial sites or participants Liege, Belgium- February 2, 2023: Adherence specialists AARDEX Group and remote research platform experts in the Clinpal team at Cambridge Cognition have teamed up

Poor medication adherence during clinical trials can skew results, leading to underestimations of product efficacy, inaccurate pharmacokinetic/pharmacodynamic calculations, and even put participant safety at risk.1 Research has shown that up to 50% of people who take part in drug development

The World Health Organization (WHO) has recently highlighted how poor adherence can lead to poorer outcomes for prisoners, contributing to health inequalities the world over. But it is worth noting that near-perfect adherence can be just as troublesome within the

Poor adherence to the investigational product can bring even the most carefully planned clinical trial to its knees. It is time to accept that traditional approaches such a pill count do not work. We need to upset the status quo

Belgium – medmix Drug Delivery (Haselmeier) and AARDEX Group announced today a collaboration, combining Haselmeier´s D-Flex™ Logbook with AARDEX Group’s Medication Adherence software and hardware ecosystem. Clinical testing during new drug development is costly and time intensive for pharmaceutical and

Poor medication adherence in clinical trials may be a long-standing challenge, but advanced approaches and technologies are finally cutting it down to size. Join AARDEX’s scientific lead at the 21st Clinical Trials Europe event to find out how. Bernard Vrijens and

Adherence to medication during clinical trials is a problem of striking magnitude, and one that researchers have employed therapeutic drug monitoring (TDM) to solve for years. But the approach, which measures the level drug or drug metabolites in patients’ blood,

Every clinical trial participant with poor medication adherence will have their own set of complex reasons for not taking their medications as prescribed – meaning there is no one size fits all solution. Instead, sponsors should take a data-driven approach

When clinical trial participants do not follow the dosing protocol, it can result in an underestimation of a drug’s therapeutic effect. With inability to demonstrate efficacy being the leading cause of study failure worldwide1, it’s an issue sponsors cannot afford

While digital pills have long been hailed as a valuable solution to the stubborn medication adherence problem, there have been questions about their use, in terms of intrusiveness, acceptance, and reliability. But with the evidence base expanding, there are opportunities

Even the most efficacious medicines do not work when people don’t take them – and the same is true for digital medication adherence monitoring. Because while the approach has huge potential in clinical trials and routine practice alike, embracing the

Digital health technologies (DHT), including digital medication adherence monitoring, have the potential to revolutionize drug development, and must be given every opportunity to succeed. That’s according to the European Federation of Pharmaceutical Industries and Associations (EFPIA) reflection paper, which has

Proving the efficacy of investigational products is a complex process but with the right Medication Adherence Technology, sponsors can generate the evidence that they need to get drugs from labs to life, faster. Unmet Medical Need Non-steroidal anti-inflammatory drugs (NSAIDs)

Drug giant, Pfizer has highlighted the need for effective, patient-centered medication adherence management in clinical trials. Nothing stops a drug from working more than not taking it, but, as medicine and leadership pioneer and Pfizer’s Chief Medical Officer, said, non-adherence

Combining BIOCORP’s pen injector add-on with AARDEX® Group’s Software to improve adherence to medication in a Hybrid Phase IV Diabetes Study. Liege, Belgium – July 19, 2022: Following the launch of their global collaboration to support medication adherence in different fields last

Effectively measuring medication adherence relies on selecting the most appropriate data source – and for clinical trials, that data source is electronic medication monitoring. The TEOS (timelines-events-objectives-source) guideline provides a clear framework on how to assess medicine-taking behavior relative to

Realizing the potential of the revolution in cancer treatment depends on ensuring researchers, regulators, and clinicians have access to all the information they need to develop, approve, and prescribe new drugs – including patient adherence data. Nothing stops a drug

Poor medication adherence is a problem that has gone “unsolved” for decades. But combining smart caps with individualized feedback can drive the adherence that proves the investigational product efficacy and underwrites approvals. In this case study, we set out the

The emergence of pharmacogenomics is an important milestone in the precision medicine journey – but the importance of measuring medicine adherence, an essential component in the complex pharmacokinetics/ pharmacodynamics equation, can often be overlooked. In this article, Bernard Vrijens, CEO

Welcome to the May edition of AARDEX Group’s Clinical Trials and Tribulations Newsletter – an aggregated collective of all of the industry’s hottest news and views! Breaking News New Pivotal Data Demonstrates the Clinical Benefit of Roche’s glofitamab, a Potential

The emergence of outcome-based contracts in clinical research has placed an additional consideration on the already thorny issue of adherence. When drug companies are paid according to the real-world effectiveness of their products, ensuring people take the medications as prescribed

If enacted, the US’ Right Drug Dose Now Act will place pharmacogenomics on the main stage of drug development and move us further into the personalized medicine era. By supporting the use of pharmacogenetic testing to prevent adverse events (AEs)

“Nothing stops a drug from working like not taking it, making patient adherence one of the most important factors to consider when designing a clinical trial.” – Bernard Vrijens, Scientific Lead, AARDEX® Group Poor patient adherence is a problem that has

Despite huge strides in how cardiovascular diseases (CVD) are treated in recent years, the conditions remain among the leading causes of morbidity and mortality worldwide. At least part of the problem is that even the most effective drugs will not

The partnership sees the integration of AARDEX’s adherence analytics software with etectRx’s ingestible adherence sensor smart pill. Liege, Belgium (APRIL 28, 2022) – Belgium-based AARDEX Group, the leader in measuring and managing medication adherence in clinical trials today announced a strategic

With patient adherence thought to be lower than 50% 1, drug companies are increasingly maximizing dosages to bridge the gap at population scale – but are we overdosing individual patients as a result? The industry’s tendency to always aim for the

Diversity in clinical research is a growing concern for sponsors as many factors can influence how an individual may react to certain drugs: age, biological sex, disabilities, chronic comorbidities, geographical location, gender identity, race, and ethnic background. Thus, if the

Decentralized Clinical Trials (DCTs) are here to stay, as demonstrated by international regulators’ recent commitments to the model. But while the approach offers a multitude of benefits, from expanding access to increasing cohort diversity, it can also serve to magnify

In a world of big tech, data is king. This slogan is far from news for the Biopharmaceutical Industry, but clinical trials are lengthy, complex, and expensive, meaning precious information isn’t always utilized to its maximum potential. In recent years,

It’s hard to believe that we are already a full quarter into 2022 – and a busy one it’s been -particularly for the regulatory landscape. AARDEX® Group also announced the first in a series of virtual events, a live panel debate

In December last year, the Food and Drug Administration (FDA) issued new draft guidance (Digital Health Technologies for Remote Data Acquisition in Clinical investigations) on what kinds of devices and software may be used for remote monitoring of clinical trial

Technology presents the healthcare community with a golden opportunity to improve lives – but only if we embrace that potential. Success, according to the Innovative Health Initiative’s Strategic Research and Innovation Agenda, depends on cross-sector collaboration. Across Europe, healthcare faces

Clinical research can stand or fall on the adherence to trial medications – even the most efficacious interventions will fail if people do not take their medicines as prescribed. That’s why researchers from the University of Sydney and the University

In recent years, a multitude of Digital Health Technologies (DHTs) have emerged, many with the ability to solve some of the biggest problems in clinical trials. However, uptake has been slow. Now, proposed new Food and Drug Administration (FDA) guidelines

For far too long, poor patient adherence has been the elephant in the room. The common, age-old problem has the potential to bring a clinical trial to its knees, and yet with no workable solution on the table, sponsors and CROs have

According to the World Health Organization, up to half of the patients with chronic diseases fail to take their medications properly.1 Research shows many patients who are part of clinical trials do not maintain medication adherence either.2 Among many things, poor patient adherence

Poor patient adherence tends to be a matter of people not taking their medicine as prescribed, due to access barriers. So how can placing an additional burden on patients be the answer? As an industry, we are more than aware

Innovative medication adherence tools in a real-world hospital setting to reduce anxiety in users of mental health services. Liege, Belgium – March 3, 2022: Belgium-based AARDEX Group, the global leader in Digital Tools for measuring and managing adherence to medication, today announced they have

Recent advances in medical science are providing new hope for people living with unmet needs – but modern medicines are only one side of the coin. Ensuring new treatments move from bench side to bedside as soon as possible is

Poor adherence to medication has been costing lives and money for decades – but cutting-edge solutions are now on hand to solve the age-old problem. It is no secret that the medical community has a problem with patient compliance: patients

It may hold the potential to solve one of healthcare’s biggest problems, but not all Medication Adherence Tools are created equal. The key to helping people to take their medicines as prescribed, whether within a clinical trial or as part

When it comes to medication adherence in psychiatry and oncology trials, the stakes are high – not taking medicines as prescribed can have life-altering or even life-threatening consequences. As such, we often assume almost perfect adherence. Unfortunately, this could not

Boost clinical trial retention with participant engagement and adherence patterns Clinical trial participants are among a study’s most valuable assets – which is why Sponsors & CROs are increasingly investing to keep them on board, on track, and on the

It’s no secret, recruiting clinical trial participants is difficult, time-consuming, and considerably expensive, all while dropout rates are notoriously high across therapeutic areas. Whatever the motive for dropout, the impact is huge. Trials can become underpowered and may not have

Drug development is inherently risky, with some analysis showing that just 13.8% of products that enter industry-sponsored Phase I trials go on to obtain FDA approval.1 Of course, the exact figure varies across therapy areas and development stages, but it is

Adherence monitoring can minimize variability and maximize engagement in DCTs Decentralized clinical trials (DCT) are growing in popularity, thanks to their ability to tear down many of the long-standing barriers to robust medical research. And while this new model has

Study power is the Holy Grail of clinical trials, setting drug developers on the quickest path to market – but non-adherence causes costly detours. When participants do not take their medication as specified in the study protocol, it can result

As we prepare to wrap up the year, we wanted to take an opportunity to reflect on the past twelve months. While COVID-19 had an unprecedented impact on our day-to-day lives, we can take solace in the fact that the

If 2020 brought the challenges of 21st-century healthcare to the fore, 2021 has been the year of building the case for a new approach. But as we look ahead to 2022, how can we take what we have learned since

Building sustainable patient-centric trials to weather the storm of COVID-19 involves the utilisation of smart connected devices and packaging to reduce the burden on patients … Read More

Our latest article in Applied Clinical Trials Online discusses the Role of Data Science and Understanding Patient Behavior. … Read More

AARDEX Group and Pill Connect Ltd Partner to Empower Patient Adherence … Read More
This article is taken from Pharmaceutical Manufacturing and Packing Sourcer February 2021, pages 46-49. © Samedan Ltd
AARDEX Group and Pill Connect Ltd Partner to Empower Patient Adherence … Read More

Bernard Vrijens our CEO and Scientific Lead, and Anna-Elisa Hein realizing a master thesis placement at AARDEX Group, conducted a study analyzing the strengths, weaknesses, threats, and opportunities of digital innovation for the personalized management of medication adherence. Anna-Elisa interviewed

We are soon leaving a very difficult year in all aspects. We lived with global pandemic and civil troubles in the news all year long. However, our Scientific Lead, Bernard Vrijens sees an uptake in remote, decentralized, and hybrid trials.

AARDEX’s Scientific Lead Bernard Vrijens and Biocorp’s business development director Arnaud Guillet give some details about their companies alliance. They explain how Biocorp’s Injay connected prefilled syringe (PFS) combined with AARDEX’s MEMS Adherence Software provides a mean to tackle the

Medication adherence, which involves patients taking medications in accordance with how they have been prescribed, is a widespread challenge, with wide-reaching implications for patients and health services worldwide. Managing patient compliance can make the difference between failed and successful clinical

AARDEX Group, the world leader in medication adherence solutions, today announced a strategic partnership with BIOCORP, a French company specialized in the design, development and manufacture of innovative medical devices. … Read More

AARDEX is proud to announce the integration of its MEMS® Adherence Software by a major pharmaceutical company. It’s the first end-to-end integration of electronically compiled dosing histories using smart packages from interactive response technology (IRT) into electronical data capture systems
MEMS® has been a part of successful adherence research for more than 30 years.Download this adherence compendium and find out how digital medication adherence monitoring increases the impact of academic research findings.

By establishing itself in the U.S., the Group aims to strengthen its business and maintain its position as market leader in digital adherence monitoring systems. Joe Keenan will lead the business development for the pharma and biotech industry in North America.

Electronic monitoring, a digital technology based on smart packages, enables the most objective and precise adherence monitoring. By using an evidence-based digital adherence monitoring system, Sponsors and HCPs improve drug efficacy by managing patient adherence to the medications. In this
50 % of patients involved in clinical trials do not take their medication as prescribed, resulting in underestimated drug efficacy and delayed approval of the investigational product. Poor medication adherence is thus a threat to drug development. Stop using unreliable

The webinar “SMART Medication Adherence Monitoring – Improving Speed and Accuracy of Clinical Trials“, held on April 15, was co-organized with our partner WestRock. Bernard Vrijens, Scientific Lead of AARDEX, has discussed the challenges of patient non adherence to medications

Recently, the FDA (Mar 2019) and the EMA (Feb 2020) have finalized guidances to monitor and mitigate the risk of patient non-adherence to the treatments tested in clinical trials. Under the auspices of the International Society for Medication Adherence (ESPACOMP), an

SRIW, Sowalfin, Noshaq and CCF (Centre de Cautionnement et de Financement du Valais) are now part of the AARDEX Adventure.

Medication adherence—the extent to which patients take medications as prescribed in agreement with their health care provider—is an ongoing public health priority, given that improved adherence can lead to better clinical outcomes. … Read More
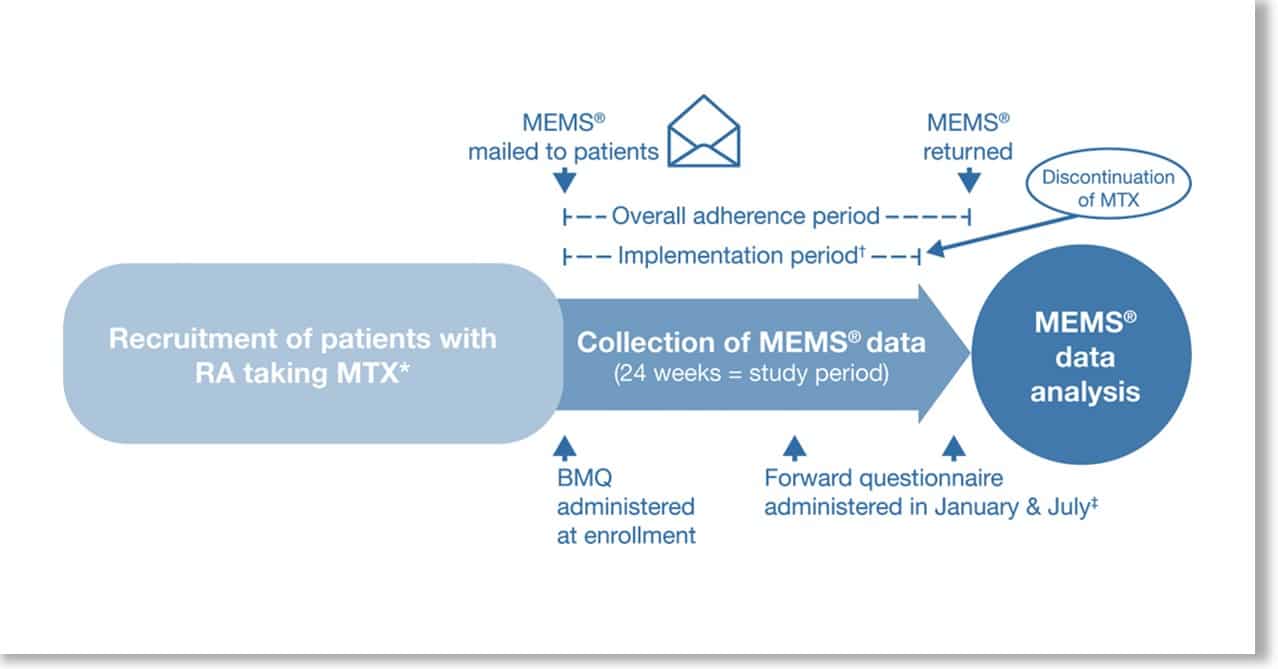

AARDEX’s scientific lead Bernard Vrijens is a co-author of the new publication in the Osteoporosis International journal about determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment. … Read More

Discover why the Pharmaceutical world trusts us to resolve patient non-compliance. … Read More

Bernard Vrijens, CEO of AARDEX Group, is invited as a guest speaker at the 2019 annual meeting of the ERS, Madrid, Spain, September 30, 2019. … Read More

Discover our CEO’s last publication in the British Journal of Clinical Pharmacology … Read More

AARDEX Group, the leader in medication adherence monitoring and management … Read More

Bernard Vrijens, CEO of AARDEX Group, is invited as a guest speaker at the 2019 … Read More
AARDEX still confirms its leading position in the B2B medication adherence market … Read More

DEC-2018 : Electronic compilation of drug dosing history data using MEMS® … Read More

DEC-2018 : Under the auspices of ESPACOMP, an international panel of experts recently … Read More

DEC-2018 : With an H-index of 138, AARDEX Group steadily grows its contribution to adherence sciences … Read More

NOV-2018 : Bernard Vrijens, CEO of AARDEX, is an invited speaker at the 42th Annual Conference … Read More

NOV-2018 : Bernard Vrijens, CEO of AARDEX, is an invited speaker at the 2nd value added medicines … Read More

OCT-2018 : AARDEX Group will be at ESPACOMP 2018 from November 29th to December … Read More

OCT-2018 : AARDEX group will speak at the Annual Meeting of the American College of Rheumatology … Read More

OCT-2018 : AARDEX Group will be at ISPOR Europe in Barcelona 10-14 November 2018. … Read More

AUG-2018 : The new MEMS® 8 technology has a precision comparable … Read More
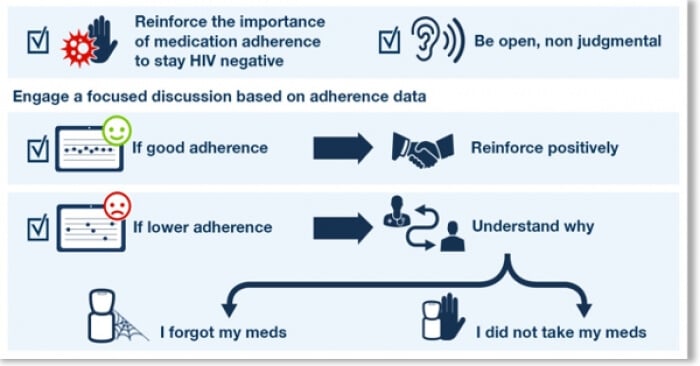
AUG-2018 : Amsterdam, 22nd International AIDS Conference: AARDEX Group presented … Read More

AUG-2018 : In this groundbreaking publication, the OECD discusses the importance of medication … Read More

JUL-2018 : The National Institutes of Health (NIH) qualified medication adherence … Read More

FEB-2018 : The Belgian-Swiss company AARDEX Group has announced the completion … Read More
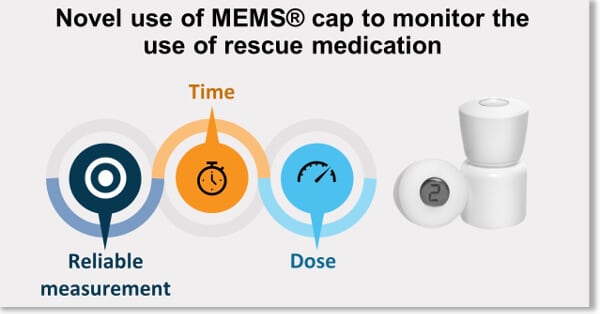
DEC-2017 : MEMS® was used successfully to show the adequate coverage … Read More
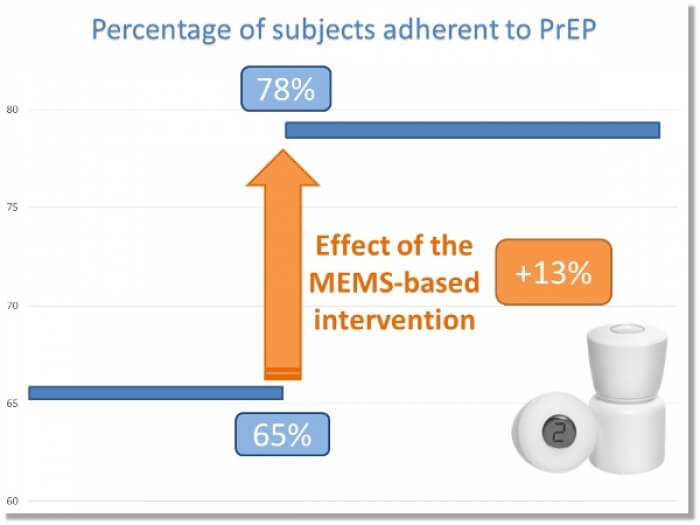
NOV-2017 : Incredible, one year has already passed since we presented one of the most … Read More
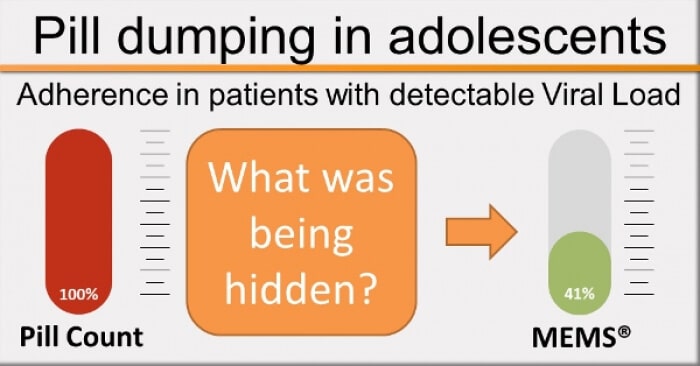
NOV-2017 : Frequently, poor-responders with high pill count adherence are dumping … Read More
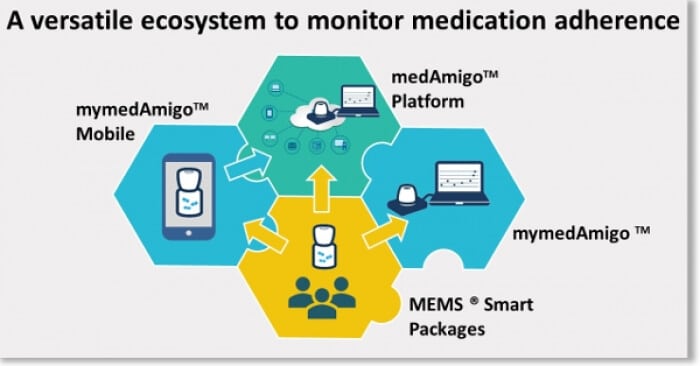
NOV-2017 : The MEMS® ecosystem is the most versatile medication adherence … Read More
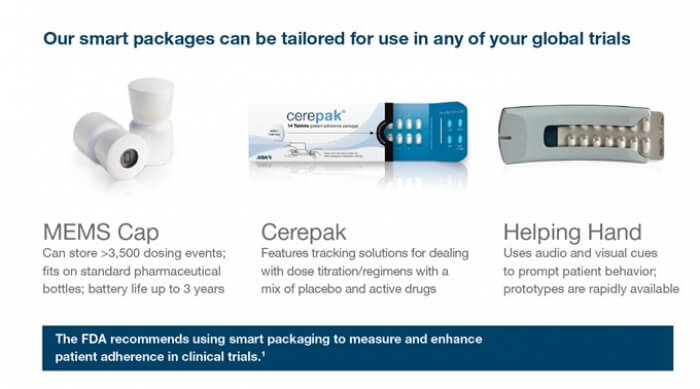
OCT-2017 : Want to meet the team behind MEMS®, the original Smart Packages … Read More

FEB-2017 : Nature Reviews on Drug Discovery features adherence-informed … Read More

Compelling data presented at the 27th edition of JESFC, Paris, January 13, 2017 … Read More

clinical studies using MEMS®. Our product bibliography for MEMS® now exceeds … Read More

Successful measurement-guided Adherence-enhancing intervention in HIV pre-exposure (PrEP) … Read More

WestRock Healthcare takes the floor at CDER Drug Packaging Summit at US Food … Read More

Best Patient-Focused Technological Development at the First Clinical and Research Excellence … Read More

FEB-2016 : High-fidelity measurement of patients’ medication adherence … Read More

OCT-2015 : Medication adherence, a key indicator … Medical adherence … Read More

DEC-2014 : Grant from The Bill & Melinda Gates Foundation to improve adherence … Read More